Fig. 1.
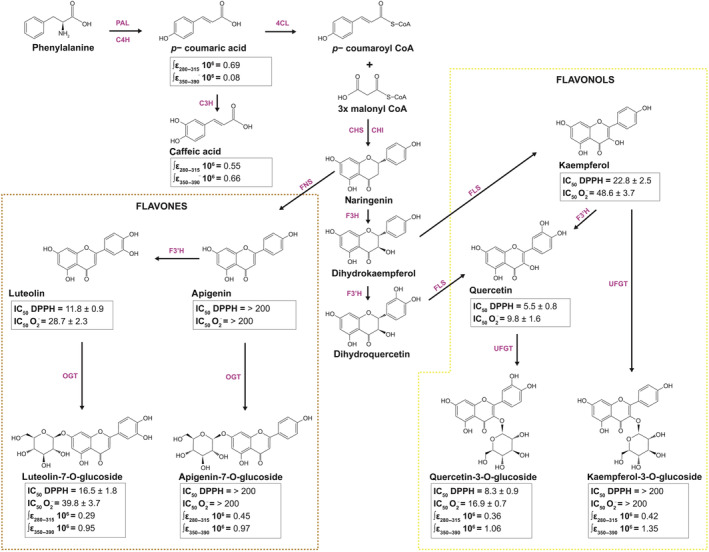
Simplified scheme of the phenylpropanoid pathway leading to the biosynthesis of hydroxycinnamic acid derivatives (HCAs, here reported are p‐coumaric and caffeic acids), mono‐ and dihydroxy B‐ring‐substituted flavones and flavonols (FLAV). The UV‐absorbing capacity of HCAs and FLAV has been measured by integrating individual molar extinction coefficients (ε) over the 280–315 (UV‐B) and 315–390 nm (UV‐A) waveband. The antioxidant capacity of FLAV, both aglycones and glycoside derivatives, has been estimated by calculating the concentration (μM) of individual metabolites capable of reducing by 50% (IC50) that of the synthetic free radical DPPH (2,2‐diphenyl‐1‐picrylhydrazyl) and the superoxide anion (O2 −), following the spectrophotometric protocols of Baratto et al. (2003). Data of IC50 are means ± SD of three replicate measurements. 4CL, 4‐coumaroyl‐CoA ligase; C3H, p‐coumarate 3‐hydroxylase; C4H, cinnamate 4‐hydroxylase; CHI, chalcone isomerase; CHS, chalcone synthase; F3′H, flavonoid 3′‐hydroxylase; F3H, flavanone 3‐hydroxylase; FLS, flavonol synthase; FNS, flavone synthase; OGT; 7‐O‐glucosyl transferase; PAL, phenylalanine ammonia‐lyase; UFGT; UDP glucose‐flavonoid 3‐O‐glucosyl transferase.
