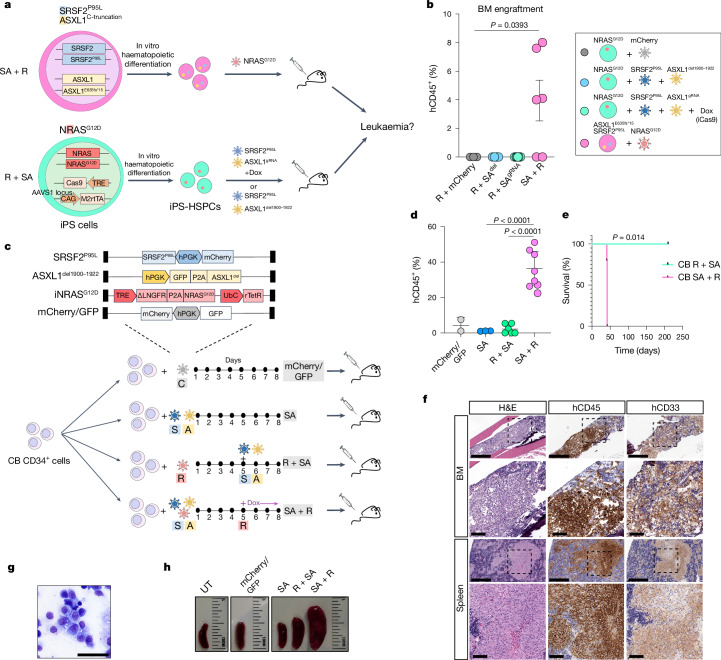
Fig. 1. RASmut are obligatory late events in AML and need to be acquired after specific cooperating mutations.
a, iPS cells with heterozygous SRSF2 and ASXL1 (top) or NRAS (bottom) mutations were differentiated into HSPCs and transduced with lentiviral vectors encoding NRASG12D (top) or SRSF2P95L and either a truncated dominant-negative ASXL1 transgene (ASXL1del1900–1922) or a gRNA targeting exon 12 of ASXL1 (bottom) and transplanted intravenously into NSGS mice. b, Human engraftment in the bone marrow of mice 13–15 weeks post-transplantation. Each data point represents one mouse: n = 3 (R + mCherry), 4 (R + SAdel), 8 (R + SAgRNA) and 6 (SA + R) from two experiments. Mean and s.e.m. are shown. P values were calculated with a two-tailed unpaired t-test. c, CB CD34+ cells were transduced with the lentiviral vectors shown at the indicated time intervals of in vitro culture, with Dox added to the culture at the indicated time point to induce NRASG12D expression. The cells were prestimulated for 4 days before and were injected into NSGS mice 7 days after the first transduction. d, Human engraftment in the bone marrow of NSGS mice transplanted with CB CD34+ cells shown in c. P values were calculated with one-way ANOVA; n = 2 (mCherry/GFP), 3 (SA), 6 (R + SA) and 8 (SA + R) mice. Mean and s.d. are shown. e, Survival of mice from c; n = 5 (SA + R) and 3 (R + SA) mice. f, Bone marrow and spleen images from a mouse transplanted with CB SA + R cells representative of at least three experiments. Left, haematoxylin and eosin (H&E) staining. Middle and right, immunohistochemistry for hCD45 (pan-haematopoietic) and hCD33 (myeloid) markers. g, Wright–Giemsa-stained human cells retrieved from the bone marrow of a mouse transplanted with SA + R CB cells. Image representative of at least three independent experiments. h, Representative images of spleens from mice transplanted as shown in c. BM, bone marrow; UT, untransplanted. Scale bars, 500 μm (f, lower magnification panels), 100 μm (f, higher magnification panels), 50 μm (g).