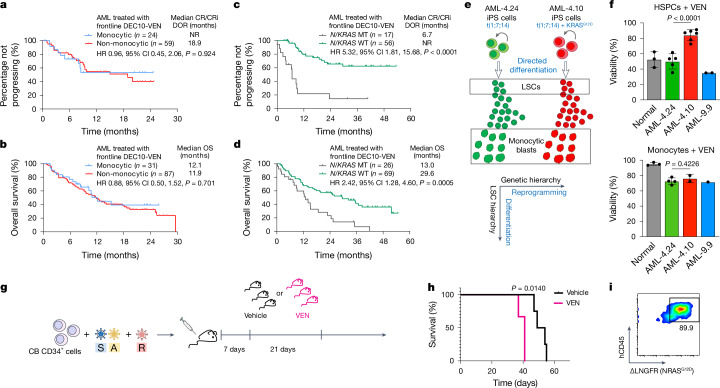
Fig. 4. RASMT LSCs drive clinical resistance to VEN.
a–d, Outcome data from 118 older or unfit patients with newly diagnosed AML treated in a prospective trial with 10-day DEC and VEN (DEC10-VEN). DOR (a) and OS (b) in patients with monocytic versus non-monocytic AML. DOR (c) and OS (d) in patients with AML with TP53-WT with versus without N/KRAS mutations. log-rank test, two-tailed unadjusted P values. e, Two iPS cell lines derived from a patient with AML, one capturing the RAS-WT major clone (AML-4.24) and one the KRASG12D subclone (AML-4.10), were differentiated in vitro to LSCs and to monocytic blasts. f, HSPCs and monocytes derived from normal iPS cells and from the indicated AML-iPS cell lines were treated with VEN and viability was measured by CellTiter-Glo. Viability compared with dimethylsulfoxide (DMSO)-treated is shown. HSPCs, n = 3 normal, 5 AML-4.24, 7 AML-4.10 and 2 AML-9.9; monocytes, n = 3 normal, 4 AML-4.24, 2 AML-4.10 and 1 AML-9.9 independent experiments; mean and s.d. are shown. P values were calculated with a two-tailed unpaired t-test. g, CB CD34+ cells transduced with SA + R, as shown in Fig. 2a were transplanted into NSGS mice. The mice were treated with VEN (100 mg kg−1 day−1 by oral gavage) or vehicle, starting 1 week post-transplant, daily, for 3 weeks. h, Survival of mice from the experiment shown in g; n = 3 (VEN) and 4 (Vehicle). P value was calculated with a log-rank (Mantel–Cox) test. i, NRASG12D expression in hCD45+ cells from the bone marrow of a moribund mouse treated with VEN. CI, confidence interval; CR, complete remission; CRi, CR with incomplete haematologic recovery; HR, hazard ratio; NR, not reached.