Abstract
Background and purpose
Previous studies demonstrated cognitive deficits in patients with peripheral vestibulopathy (PVP) with dysfunction of spatial navigation and orientation, but also documented cognitive decline in nonspatial abilities. This study evaluates cognitive deficits in patients with unilateral vestibulopathy (UVP) as well as bilateral vestibulopathy (BVP) in multiple cognitive domains using common screening tests to reliably detect these deficits in clinical practice.
Methods
This prospective study compared patients with UVP and BVP to age‐ and sex‐matched healthy controls (HC). Tests included the Alzheimer's Disease Assessment Scale (ADAS), Mini‐Mental Status Examination (MMSE), Trail Making Test Part A and B, Clock Drawing Task, Executive Interview‐25 (EXIT25), Dementia Detection (DemTect), and the Judgment of Line Orientation (JLO). The Montgomery‐Åsberg Depression Rating Scale was used to control for depression. Videonystagmography objectively reconfirmed PVP. The Vertigo Symptoms Scale and the Dizziness Handicap Inventory were used to assess for symptom severity and restrictions of activities of daily living.
Results
Eighty‐one patients (65 UVP, 16 BVP) were compared to 55 HC. Patients showed impairment in ADAS, MMSE, DemTect, EXIT25, and JLO. No differences between UVP and BVP were detected. The relative risk (RR) estimates of developing cognitive deficits following PVP were increased. The RR for the ADAS was higher in BVP (RR = 4.91, 95% confidence interval [CI] = 1.87–12.9, p = 0.001) than in UVP (RR = 3.75, 95% CI = 1.65–8.51, p = 0.002), but was similar for the MMSE and DemTect between groups.
Conclusions
Patients with PVP showed deficits in multiple cognitive domains including nonspatial cognitive abilities. Vestibulopathy could be a risk factor for the development of cognitive impairment.
Keywords: cognitive deficit, dementia, nonspatial abilities, peripheral vestibulopathy, screening tests
INTRODUCTION
Peripheral vestibular disorders (PVDs) are common across all age groups, with an estimated prevalence of 6.5% (6461/100,000 individuals) in the general population [1]. The prevalence of PVDs increases with age and has a considerable impact on health care systems. The primary function of the vestibular system is to maintain balance and gaze stabilization. The vestibulo‐ocular reflex stabilizes the visual world during movement and is considered the fastest reflex in the human body, whereas the vestibulospinal reflexes enable the body to retain its vertical body alignment during dynamic motion [2]. More than any other sensory system, the vestibular system employs widespread projections to many subcortical and cortical areas, including those involved in autonomic functions, emotions, sleep, and cognition [3]. These connections are considered among the oldest evolutionary neuronal systems. A growing body of evidence suggests that dysfunction of the vestibular apparatus or its connecting nerve not only has serious implications for its primary function, but may also interfere with higher cortical abilities [4, 5, 6, 7, 8, 9, 10]. It was demonstrated that the connections to the hippocampal formation and medial temporal lobe are paramount for the cognitive impairment of spatial memory and navigation in patients with vestibular dysfunction [7, 8]. It was suggested that other cognitive domains may also be impaired and that there may even be a causal relationship between peripheral vestibular loss and dementia [2, 11, 12, 13]. A cross‐sectional study has found increased prevalence of Alzheimer disease (AD) in individuals with bilateral vestibulopathy (BVP) [6]. Although a direct connection to AD remains unconfirmed, numerous studies in the past have shown general cognitive deficits in patients with vestibulopathy, including executive function, visuospatial abilities, attention, and short‐term memory [14, 15].
The evidence of cognitive deficits beyond the spatial domain is assertive, but the discussion of which cortical domains are generally affected is still ongoing, as results between very different study designs show large variations. The screening tools used to detect these cognitive deficits in patients were also very diverse and often did not resemble those psychological tests generally used in a routine clinical setting, although they were generally well established. This makes a comparison very difficult and does not provide guidance for neuropsychological screening in clinical practice.
Our objective was to test for cognitive performance deficits in the main cognitive domains including executive function, attention, working memory, episodic memory, psychomotor speed, and accuracy in patients with UVP and BVP using common cognitive tests that can identify these cortical deficits reliably and within an acceptable time frame in routine clinical practice.
METHODS
Participants
Patients with unilateral vestibulopathy (UVP) or BVP were recruited prospectively over 1 year from the outpatient clinic of a tertiary dizziness and vertigo center (Figure 1, Consort Statement). The study was approved by the Ethics Committee of the University of Duisburg‐Essen. Informed written consent was obtained from all participants prior to participation. Clinical assessment included neuro‐otological, neurological, and physical examination, complete medical history, and bithermal water caloric testing in a supine position. Warm (44°C) and cold (30°C) water irrigations of at least 250 mL were administered for 30 s. Eye movements were recorded with videonystagmography (Interacoustics). A side difference of ≥50% slow phase velocity (SPV) was considered pathologic in UVP, and the sum of bilateral caloric response of <20°/s SPV in BVP. Both patient groups had to have a pathological head impulse test on clinical examination. There were no clinically identifiable hearing impairments, but formal auditory testing was not performed.
FIGURE 1.
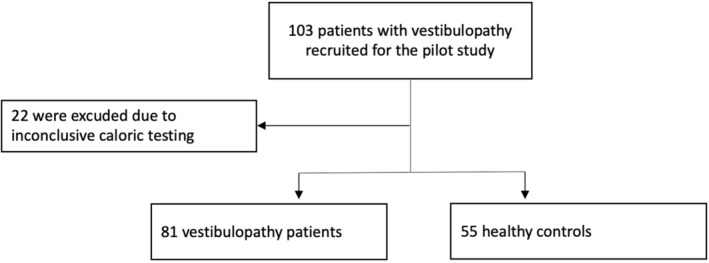
Study recruitment CONSORT (Consolidated Standards of Reporting Trials) statement.
Neuropsychological tests
The following neuropsychological tests were performed in a face‐to‐face interview setting with every participant to assess a wide variety of cognitive domains, but also to identify potential screening tests that would allow easy detection of cognitive deficits in patients with peripheral vestibulopathy in clinical practice.
Alzheimer's Disease Assessment Scale
The Alzheimer's Disease Assessment Scale (ADAS) is a composite of individual and independently valid measures that have each been rescaled. The ADAS test has been validated for use in patients with AD and many of the cognitive domains involved in dementia. It is a very sensitive psychometric scale for measuring cognitive function. A score of 12 or higher was considered pathologic [16].
Mini‐Mental Status Examination
The Mini‐Mental Status Examination (MMSE) is a brief, 30‐item test of cognitive function that has been extensively used since it was developed in 1975. Items evaluate orientation, short‐term memory (registration), recall, attention, calculation, and language. Lower scores reflect worse cognitive ability. It is a screening test for cognitive deficits. Cutoff score from normal to cognitive impairment is 26 points [17].
Dementia Detection
The Dementia Detection (DemTect) is a short, easy to administer, highly sensitive psychometric screening test to identify patients with mild cognitive impairment and early stage dementia independent of age and education. Its transformed total score is 18. Cognitive impairment is considered at score 12 or lower. The test is carried out in the form of an oral and written questionnaire, and the patient's performance is recorded on a test sheet by the examiner. Tested are functions of verbal memory, fluency, intellectual flexibility, and attention. It only takes about 8–10 min to administer. This test was used in a large number of scientific studies and is generally considered to have very good validity and psychological test values. Its sensitivity to detect cognitive deficits was reported to be as high as 97%, with a specificity of 93% [18].
Trail Making Test
In the mid‐1940s, the American Army incorporated the Trail Making Test (TMT) into the Army as the Individual Test of General Ability and gave it its current name. It had existed in other forms before this and had always been considered a test of attention. Its purpose includes the detection of frontal lobe deficits, problems with psychomotor speed, visual search and sequencing, and attention, and impairments in set‐shifting. The test uses the first 12 letters of the alphabet and Arabic numerals 1–25. It consists of Part A and Part B. Part A includes numbers only. Part B includes numbers and letters and requires the patient to shift between consecutive numbers and letters. The score, up to a maximum number of seconds, is the time for the patient to complete the task, with higher times reflecting increasing impairment. A time of 180 s or more was considered pathologic [19].
CLOX
The CLOX is a clock drawing task that is designed to elicit executive impairment and discriminate from nonexecutive constructional impairment. The CLOX is divided into two parts (CLOX1 and CLOX2) to help discriminate the executive control of clock drawing from clock drawing itself. In CLOX1, the patient is instructed to draw a clock that says 1:45 entirely from recall. The second part of the test (CLOX2) requires the patient to observe the examiner drawing a clock in a circle on a page, adding in the places for 12, 6, 3, and 9 first and then setting the hands to 1:45. The patient is then asked to copy what the examiner has just completed. The difference between parts 1 and 2 is hypothesized to reflect the specific contribution of executive control versus visuospatial praxis to overall clock drawing performance. Each CLOX subtest is scored on a 15‐point scale. Low scores reflect impairment. A cutoff of 10/15 was considered pathologic [20, 21].
Executive Interview‐25
The Executive Interview‐25 (EXIT25) is a brief, clinic‐based, reliable, and valid instrument for the assessment of executive cognitive function. It is a 15‐min, 25‐item interview scored from 0 to 50 (higher scores indicate greater impairment in executive control). Advantages of the EXIT25 over traditional measures of executive function include its simplicity and clinical face validity. The EXIT25 correlates well with other measures of executive function. A score of 15 or higher was considered pathologic [22].
Judgment of Line Orientation
The Judgment of Line Orientation (JLO) test was developed to be “as pure a measure of one aspect of spatial thinking as could be conceived.” Two partial line segments are presented together on one page, and the examinee is asked to match the orientation of these segments to those on a multiple‐choice response card. The response options are made up of 11 full lines, all 18 degrees apart from one another, arranged in a semicircle. The stimulus lines—partial line segments—represent either the proximal (low), middle, or distal (high) segment (one third) of the full lines. The examinee is presented with five sample items, on which erroneous responses are corrected, followed by 30 test items presented without feedback. A score of 13 or higher was considered pathologic [23].
Montgomery‐Åsberg Depression Rating Scale
The Montgomery‐Åsberg Depression Rating Scale was designed as a sensitive measure of change in the treatment of depression. It consists of 10 items such as treatment of depression, apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, an ability to feel pessimistic thoughts, and suicidal thoughts. This scale is the most widely used in drug treatment in young and older patients. Score ranges between 0 and 60. Higher scores indicate increasing severity of depression. Scores of ≥20 indicate depression that is severe enough that treatment should be considered (20–34: moderate depression; 35–60: severe depression) [24].
Self‐report vertigo and dizziness measures
The Vertigo Symptoms Scale (VSS) was used to quantify the intensity of the experienced vestibular symptoms [25]. The VSS is a 15‐item, self‐report instrument that measures the frequency of vertigo, dizziness, unsteadiness, and concomitant autonomic/anxiety symptoms over the past month. The total score ranges from 0 to 60 points, and a higher score indicates a higher frequency of symptoms. There are two subscales, the vestibular‐balance subscale and the autonomic‐anxiety subscale.
The impact of dizziness on daily life was investigated by the Dizziness Handicap Inventory (DHI) [26]. The DHI is a 25‐item self‐report questionnaire that quantifies the impact on daily life by measuring self‐perceived handicap. Item scores are summed. There is a maximum score of 100 (28 points for physical, 36 points for emotional, and 36 points for functional). Patients with a score of 0–30 points are not or mildly affected, patients with a score of 31–60 are moderately affected, and patients with a score of 61–100 points are severely affected.
Statistical analyses
Patients and demographic characteristics were assessed descriptively to compare the considered groups. Correlation of categorical variables was assessed using the Spearman correlation coefficient.
Relative risk (RR) estimates and corresponding 95% confidence intervals (CIs) were obtained via Poisson regression. Odds ratio estimates were determined on the basis of logistic regression analyses.
Comparison of cognitive deficits was conducted via unpaired two‐sample Wilcoxon tests. To study the relation of influence of age, depression, and disease duration, linear regression analysis was applied.
RESULTS
Patients and demographic characteristics
One hundred three patients were recruited; 22 patients were excluded from the study due to an inconclusive caloric test result using videonystagmography. Therefore, 81 patients were compared to 55 healthy controls in the final analysis (Figure 1, Table 1). The majority of patients had UVP (n = 64), with slightly more women (n = 36) compared to men (n = 29). The BVP group (n = 16) had more men (n = 10). The mean age did not differ between patient groups and healthy controls (Table 1). The mean duration of dizziness and vertigo symptoms was 5.15 ± 59 months.
TABLE 1.
Demographics and clinical characteristics.
| Characteristic | Healthy controls | All vestibulopathy | Unilateral vestibulopathy | Bilateral vestibulopathy | p |
|---|---|---|---|---|---|
| Mean age, years ± SD | 60.71 ± 11.61 | 63.47 ± 11.08 | 64.35 ± 11.01 | 59.88 ± 10.97 | 0.267 |
| Male, n | 16 | 39 | 29 | 10 | N/A |
| Female, n | 32 | 42 | 36 | 6 | N/A |
| Mean disease duration, months ± SD | N/A | 5.15 ± 6.98 | 5.05 ± 6.56 | 5.59 ± 8.75 | 0.547 |
| VSS ± SD | N/A | 25.53 ± 12.91 | 25.65 ± 12.64 | 25.06 ± 14.36 | 0.231 |
| DHI ± SD | N/A | 15.20 ± 11.31 | 14.78 ± 11.44 | 16.88 ± 10.98 | 0.134 |
| SPV total, °/s ± SD | N/A | 51.29 ± 35.45 | 58.62 ± 33.66 | 11.57 ± 7.22 | N/A |
| SPV affected side, °/s ± SD | N/A | N/A | 15.34 ± 13.42 | N/A | N/A |
| SPV nonaffected side, °/s ± SD | N/A | N/A | 43.28 ± 24.54 | N/A | N/A |
| MADRS | N/A | 6.11 ± 7.40 | 5.92 ± 7.43 | 6.88 ± 7.48 | 0.094 |
Abbreviations: DHI, Dizziness Handicap Inventory; MADRS, Montgomery‐Åsberg Depression Rating Scale; N/A, not applicable; SPV, slow phase velocity; VSS, Vertigo Symptoms Scale.
Neuropsychological tests
Patients with peripheral vestibulopathy showed a significant difference in the ADAS, MMSE, DemTect, EXIT25, and JLO (Figure 2, Table 2). No differences were found in the TMT or the CLOX test. There were no differences between patients with UVP and BVP in any of the investigated tests. No significant correlations of cognitive test results with the results of the caloric testing (warm or cold) SPV could be detected.
FIGURE 2.
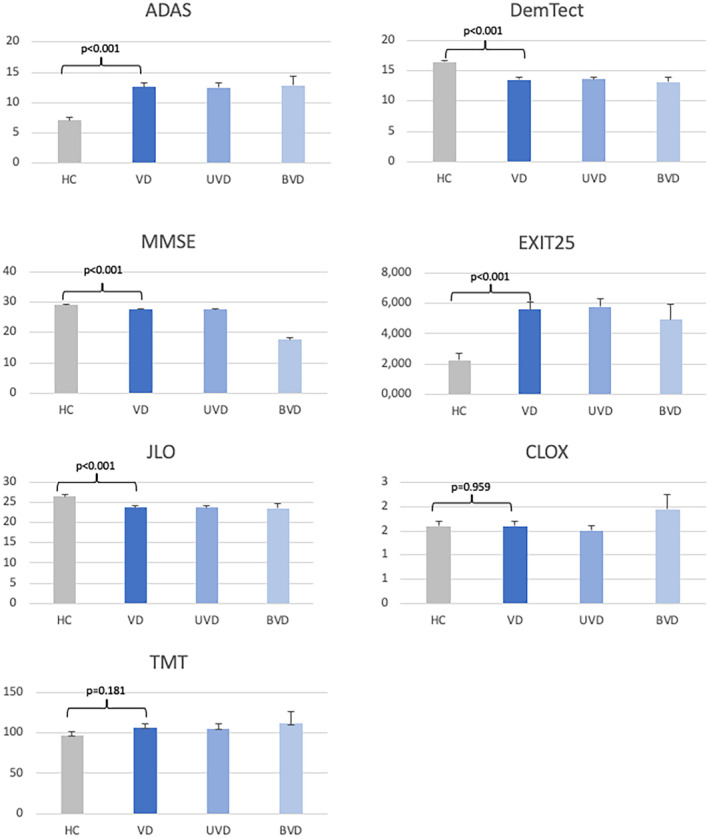
Neuropsychological test results comparing healthy controls (HC) and patient groups. ADAS, Alzheimer's Disease Assessment Scale; BVD, bilateral VD; DemTect, Dementia Detection; EXIT25, Executive Interview‐25; JLO, Judgment of Line Orientation; MMSE, Mini‐Mental Status Examination; TMT, Trail Making Test; UVD, unilateral VD; VD, vestibular dysfunction.
TABLE 2.
Neuropsychological test results.
| Test | HC ± SD | VP ± SD | UVP ± SD | BVP ± SD | VP‐HC ± SD | p |
|---|---|---|---|---|---|---|
| CLOX | 1.600 ± 0.102 | 1.593 ± 0.104 | 1.508 ± 0.103 | 1.938 ± 0.309 | −0.007 ± 0.146 | 0.959 |
| ADAS | 7.091 ± 0.486 | 12.568 ± 0.682 | 12.508 ± 0.771 | 12.812 ± 1.498 | 5.477 ± 0.837 | 0.000 |
| DemTect | 16.436 ± 0.293 | 13.525 ± 0.363 | 13.623 ± 0.403 | 13.125 ± 0.861 | −2.912 ± 0.467 | 0.000 |
| MMSE | 29.200 ± 0.143 | 27.691 ± 0.233 | 27.692 ± 0.255 | 17.688 ± 0.583 | −1.509 ± 0.273 | 0.000 |
| TMT | 97.000 ± 4.519 | 106.420 ± 5.393 | 105.123 ± 5.74 | 111.688 ± 14.562 | 9.420 ± 7.036 | 0.181 |
| JLO | 26.491 ± 0.526 | 23.704 ± 0.511 | 23.723 ± 0.591 | 23.625 ± 1.008 | −2.787 ± 0.734 | 0.000 |
| EXIT25 | 2.236 ± 0.451 | 5.605 ± 0.486 | 5.769 ± 0.555 | 4.938 ± 1.002 | 3.369 ± 0.663 | 0.000 |
Note: Level of significance: p < 0.05.
Abbreviations: ADAS, Alzheimer's Disease Assessment Scale; BVP, bilateral vestibulopathy; DemTect, Dementia Detection; EXIT25, Executive Interview‐25; HC, healthy controls; JLO, Judgment of Line Orientation; MMSE, Mini‐Mental Status Examination; TMT, Trail Making Test; UVP, unilateral vestibulopathy; VP, vestibulopathy.
RR estimates
Patients with peripheral vestibulopathy had an increased risk of cognitive performance deficits as detected by the ADAS (RR = 3.98, 95% CI = 1.78–8.87, p = 0.001), the DemTect (RR = 7.02, 95% CI = 2.15–22.95, p = 0.001), and the MMSE (RR = 10.19, 95% CI = 1.35–77.11, p = 0.025). The JLO missed the level of significance by a very slim margin and showed a robust trend (RR = 2.04, 95% CI = 0.996–4.17, p = 0.051).
The risk estimate for the ADAS was higher in the BVP group (RR = 4.91, 95% CI = 1.87–12.90, p = 0.001) compared to the UVP group (RR = 3.75, 95% CI = 1.65–8.51, p = 0.002). There was no marked difference between the two patient groups for the other neuropsychological tests (Table 3).
TABLE 3.
Relative risk estimates.
| Relative risk | 95% CI | p | |
|---|---|---|---|
| All vestibulopathy vs. healthy controls | |||
| CLOX | 0.981 | 0.419–2.295 | 0.964 |
| ADAS | 3.977 | 1.784–8.865 | 0.001 |
| DemTect | 7.016 | 2.145–22.951 | 0.001 |
| MMSE | 10.185 | 1.345–77.106 | 0.025 |
| TMT | 5.235 | 0.655–41.853 | 0.119 |
| JLO | 2.037 | 0.996–4.167 | 0.051 |
| Unilateral vestibulopathy vs. healthy controls | |||
| CLOX | 0.752 | 0.29–1.949 | 0.558 |
| ADAS | 3.747 | 1.65–8.51 | 0.002 |
| DemTect | 7.051 | 2.129–23.354 | 0.001 |
| MMSE | 10.154 | 1.32–78.09 | 0.026 |
| TMT | 4.077 | 0.476–34.897 | 0.200 |
| JLO | 2.031 | 0.971–4.246 | 0.060 |
| Bilateral vestibulopathy vs. healthy controls | |||
| CLOX | 1.910 | 0.64–5.698 | 0.246 |
| ADAS | 4.911 | 1.869–12.901 | 0.001 |
| DemTect | 6.875 | 1.719–27.49 | 0.006 |
| MMSE | 10.312 | 1.073–99.141 | 0.043 |
| TMT | 9.937 | 1.034–95.536 | 0.047 |
| JLO | 2.063 | 0.75–5.675 | 0.161 |
Abbreviations: ADAS, Alzheimer's Disease Assessment Scale; CI, confidence interval; DemTect, Dementia Detection; JLO, Judgment of Line Orientation; MMSE, Mini‐Mental Status Examination; TMT, Trail Making Test.
Influences of age, depression, and disease duration
For each year of age, the ADAS score increased by 0.184 (p < 0.001) in the healthy control group. In the vestibulopathy patients, this measure was higher at 5.08 (p = 0.001) points per year. The adjusted coefficient of determination was R 2 = 0.30. The EXIT25 showed a trend for an influence of age with a correlation of 0.207 (p = 0.064). All other tests did not show a significant correlation to age.
Depression did not have an impact on the test results of any applied neuropsychological test in our investigated groups. The DHI as well as the VSS did not show any significant differences between patients with unilateral and bilateral peripheral vestibulopathy (Figure 3). Descriptive statistics on the influence of the VSS and DHI on cognitive tests indicate that increased impairment by vertigo symptoms may be related to reduced spatial cognitive abilities as reflected by the JLO and TMT tests, although this does not reach the level of significance.
FIGURE 3.
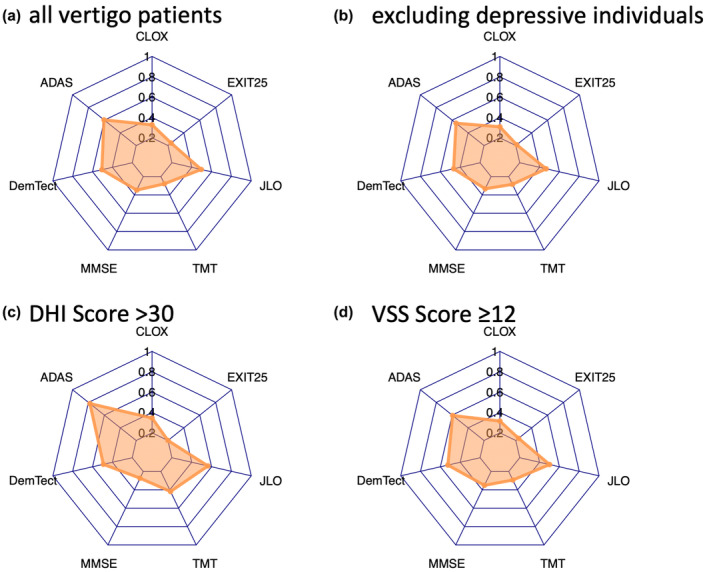
Spiderweb plots of (a) all vertigo patients, and showing influences of (b) depression, (c) Dizziness Handicap Inventory (DHI), and (d) Vertigo Symptoms Scale (VSS). ADAS, Alzheimer's Disease Assessment Scale; DemTect, Dementia Detection; EXIT25, Executive Interview‐25; JLO, Judgment of Line Orientation; MMSE, Mini‐Mental Status Examination; TMT, Trail Making Test.
Disease duration only influenced the MMSE results. Logistic regression analysis showed an odds ratio of 1.14 (95% CI = 1.00–1.29, p = 0.046) for worse MMSE score results with longer disease duration. All other tests remained unaffected by disease duration.
DISCUSSION
Patients with peripheral vestibulopathy have deficits in multiple cognitive domains including nonspatial cognitive abilities compared to healthy controls. These deficits could be detected with the ADAS, MMSE, DemTect, EXIT25, and JLO, whereas the TMT as well as the CLOX test did not show significant differences. There were no differences between bilateral and UVP detected using our test battery. The ADAS, the MMSE, and the DemTect showed different RR estimates between groups, with only the ADAS showing a 1.5‐fold risk increase of developing cognitive deficits in the BVP group as compared to the UVP group. The RR estimate for the JLO showed a trend missing the level of significance (p = 0.051). Spatial thinking is the cognitive domain that is most likely to be affected by vestibular dysfunction. Several studies described this association before more cognitive domains were investigated [8, 14, 15, 27, 28, 29]. Human studies used a virtual version of the Morris Water Task to investigate patients with vestibular failure and were able to demonstrate navigational impairment in complete as well as incomplete BVP [7]. Earlier experiments were able to demonstrate difficulties with simple path integration tasks in these patients, and pathophysiological studies could link these deficits to atrophy of the hippocampus when compared to healthy individuals. This was demonstrated in BVP as well as UVP patients [7, 8]. These results were able to reconfirm growing evidence derived from animal studies. More recent studies found deficits in additional cognitive domains such as executive function short‐term memory, processing speed, and visuospatial abilities [2].
There was no substantial influence of disease duration on cognitive deficit except for the MMSE in our study. This confirms an earlier study that also did not detect any correlation of disease duration in UVP as well as BVP to cognitive performance [9]. The authors suggested that the cognitive impairment might develop early after the dysfunction of the vestibular system. In the Baltimore Longitudinal Study of Aging, the MMSE was highly correlated with vestibular dysfunction as measured by vestibular evoked myogenic potentials, when age, sex, race, education, cardiovascular risk factors, vision, and hearing were included in the statistical model. When the last three factors were taken out of the analysis, the association with the MMSE vanished [30], whereas other cognitive performance test such as Card Rotations, Purdue Pegboard, Benton Visual Retention Test, and TMT B continued to show an association with vestibular function in both models. This was interpreted to reflect deficits in visuospatial acuity, whereas executive function and verbal memory remained widely intact [30]. This is interesting, as the TMT was one of only two cognitive tests in our study that did not seem to be affected by peripheral vestibulopathy compared to healthy controls, whereas we did find a difference in the MMSE in our patients. The reasons for these discordant findings remain unclear, but it can be hypothesized that a control group is very important in this matter and that larger, longitudinal studies would help to understand the timing and the mechanisms associated with the development of cognitive deficits following vestibulopathy and its relationship to age.
The Baltimore Longitudinal Study of Aging reported strong associations between age and vestibular decline as measured by vestibular‐evoked myogenic potentials, as well as visuospatial abilities, working memory, and attention [30]. The only neuropsychological test that was influenced by age was the ADAS in our study. This was the case for the healthy control group, but much more pronounced in the vestibulopathy patients, with a 25‐times higher increase of the ADAS score per year compared to healthy individuals, although they were age‐matched. The influence of age especially in this patient group must be kept in mind in clinical practice, but also in the planning and conduction of future studies.
There are a number of studies connecting vestibular loss to aging and linking this combination to a higher risk of developing cognitive deficits and even dementia. Some authors suggested the hypothesis that vestibular loss may contribute to a “spatial” subtype of AD on the basis that older adults with declining vestibular function often develop poorer spatial cognitive skills, including mental rotation, spatial memory, and spatial navigation, similar to what can often be observed in AD [2, 30]. Interestingly, patients with AD showed twice the level of vestibular impairment as healthy older adults, hinting at a mutual influence of dementia and vestibular dysfunction [30, 31]. Vestibular dysfunction worsens cognitive function in AD, and AD worsens vestibular function more than would be expected in an otherwise healthy elderly population. Although this mutual influence seems to be most obvious in the spatial cognitive domain, it most likely is not limited to it [6]. Hippocampal atrophy was suggested to represent the neuroanatomic correlate of reduced spatial cognitive abilities associated with vestibular impairment and is also generally considered to be one of the primary pathophysiological correlates of AD [7]. The peripheral vestibular system projects to cholinergic neurons in the medial temporal region, which suggests that a potential degradation of these neurons may play a role in the development of AD [12]. However, the true magnitude of the vestibular impairment–AD connection remains unclear, especially as disease duration of peripheral vestibulopathy does not seem to have a considerable impact. A large study investigated 98 AD patients, but was unable to find an association between vestibular function and A‐beta deposition using amyloid C‐11‐labeled Pittsburgh compound B positron emission tomography imaging [32]. Future studies will have to reevaluate this connection using other, potentially more sensitive measures or direct cerebrospinal fluid analysis. It remains to be determined whether the reverse assumption, that treatment for peripheral vestibulopathy [33], such as vestibular rehabilitation therapy, may be able to positively influence cognitive deficits in these patients and in patients with mild cognitive impairment or dementia in general [2].
Concomitant depressive symptoms or severity of vertigo symptoms as detected by the VSS, as well as impairment of managing everyday activities as investigated by the DHI, had no significant impact on the results of the performed neuropsychological test battery compared to healthy controls. This was unexpected, as it is well known that depression has a negative effect on cognition and that patients with dizziness are prone to develop depression and other psychological symptoms in association with their disorder [34]. However, there are several studies that described a similar discrepancy in patients with peripheral vestibulopathy [10, 35] and one recent study was able to demonstrate a difference between younger and older patients with dizziness [36]. According to those findings, older individuals (≥60 years) had fewer depressive symptoms (mean Hospital Anxiety and Depression Scale depression score = 5.8 ± 3.6 vs. 6.5 ± 4.1) compared to younger patients [36]. This would explain our finding at least to a certain extent, as our mean age was >60 years in all investigated groups, except for the BVP group, where it was slightly lower at 59.88 years.
The DHI and VSS scores were not very high in our patients, so that they still had considerable symptoms that led them to seek professional medical care, but they obviously learned to cope with their symptoms in their everyday lives. This is not uncommon and was reported in other clinical studies as well [34]. This may pose a bias in our results toward the less affected and better off peripheral vestibulopathy patients, but also seems to be a valid compromise for a clinical study, as patients must be able to perform the neuropsychological test battery, the vestibular testing, and the clinical examinations during 1 day to participate in the study at all. The weak association of VSS and DHI severity with spatial cognitive tests such as the JLO could have turned out to be significant with more severely affected patients.
Some authors tried to explain the wide array of cognitive deficits with the inability of patients with peripheral vestibulopathy to compensate for their vestibular deficit and perform well on neuropsychological testing at the same time [37]. This compensation model states that patients use a considerable proportion of their cognitive effort to compensate for their vestibular pathology, leaving less capacity for other cognitive tasks, thus exhibiting a false cognitive decline that is explained by lack of coping capacity rather than genuine cognitive degeneration [37]. We cannot rule out this explanation completely, but also did not find any confirmation in our data, except that patients were considerably well adapted to their vestibular symptoms, with mostly moderate DHI and VSS scores. Whether this is enough to explain the marked cognitive performance deficits or contributes to the overall magnitude of cognitive decline remains to be determined in future studies.
Further limitations of this study must be addressed. We did not perform a formal hearing test, so that cognitive deficits that may be associated with impaired hearing that may have worsened with age could not be detected. We also did not perform a saccular function test but relied solely on warm and cold caloric testing using videonystagmography, thus disregarding one part of the vestibular system. Healthy controls were evaluated only clinically by an experienced clinician without formal vestibular testing. Although no healthy control complained about vestibular symptoms, showed abnormal neurological and neuro‐otological examination, or had any medical history regarding central or peripheral vestibular disorders, we cannot rule out completely that we missed someone with past peripheral vestibulopathy that was fully compensated. Although we matched for age and gender in our relatively large sample size, the healthy control group remained slightly smaller and was comprised of fewer male participants in relation to the patient groups. We did control for major confounding factors, but we cannot rule out minor influences of the abovementioned factors, so that these limitations should be kept in mind when interpreting our data.
CONCLUSIONS
Our study supports the hypothesis that the vestibular system contributes to cognitive function in humans well beyond spatial cognition and navigation. Peripheral vestibular dysfunction may be a risk factor for the development of cognitive impairment. The RR can be estimated using the ADAS, DemTect, or MMSE. The latter two tests are readily available, fast, and easy to interpret tests, suitable for everyday clinical practice. Further studies are needed to investigate the complicated underlying mutual mechanisms of vestibular dysfunction and cognition in the future.
Funding information
This study was funded by Heel, Baden‐Baden, Germany.
CONFLICT OF INTEREST STATEMENT
M.O. has received scientific support and/or honoraria from Biogen Idec, Novartis, Sanofi‐Aventis, Genzyme, Pfizer, Teva, and Eli Lilly. He has received research grants from Allergan, Electrocore, Heel, and the German Ministry for Education and Research (BMBF). B.S. is employed at Heel. H.‐C.D. has received honoraria for participation in clinical trials, contribution to advisory boards, or lectures from Böhringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Novartis, Pfizer, and Weber & Weber; and has received research support from Boehringer Ingelheim. His research has been supported by the German Research Council and the BMBF. None of the other authors has any conflict of interest to disclose.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to Achim Dörre, who supported us with the statistical methods and data analysis. Open Access funding enabled and organized by Projekt DEAL.
Obermann M, Gebauer A, Arweiler‐Harbeck D, et al. Cognitive deficits in patients with peripheral vestibular dysfunction. Eur J Neurol. 2025;32:e15907. doi: 10.1111/ene.15907
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Hülse R, Biesdorf A, Hörmann K, et al. Peripheral vestibular disorders: an epidemiologic survey in 70 million individuals. Otol Neurotol off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2019;40(1):88‐95. [DOI] [PubMed] [Google Scholar]
- 2. Agrawal Y, Smith PF, Rosenberg PB. Vestibular impairment, cognitive decline and Alzheimer's disease: balancing the evidence. Aging Ment Health. 2020;24(5):705‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hitier M, Besnard S, Smith PF. Vestibular pathways involved in cognition. Front Integr Neurosci. 2014;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bigelow RT, Semenov YR, Trevino C, et al. Association between visuospatial ability and vestibular function in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2015;63(9):1837‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Semenov YR, Bigelow RT, Xue Q‐L, du Lac S, Agrawal Y. Association between vestibular and cognitive function in U.S. adults: data from the National Health and nutrition examination survey. J Gerontol A Biol Sci Med Sci. 2016;71(2):243‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harun A, Semenov YR, Agrawal Y. Vestibular function and activities of daily living: analysis of the 1999 to 2004 National Health and Nutrition Examination Surveys. Gerontol Geriatr Med. 2015;1:2333721415607124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brandt T, Schautzer F, Hamilton DA, et al. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain J Neurol. 2005;128(Pt 11):2732‐2741. [DOI] [PubMed] [Google Scholar]
- 8. Kremmyda O, Hüfner K, Flanagin VL, et al. Beyond dizziness: virtual navigation, spatial anxiety and hippocampal volume in bilateral vestibulopathy. Front Hum Neurosci. 2016;10:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popp P, Wulff M, Finke K, Rühl M, Brandt T, Dieterich M. Cognitive deficits in patients with a chronic vestibular failure. J Neurol. 2017;264(3):554‐563. [DOI] [PubMed] [Google Scholar]
- 10. Lucieer FMP, Van Hecke R, van Stiphout L, et al. Bilateral vestibulopathy: beyond imbalance and oscillopsia. J Neurol. 2020;267(Suppl 1):241‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanes DA, McCollum G. Cognitive‐vestibular interactions: a review of patient difficulties and possible mechanisms. J Vestib Res. 2006;16(3):75‐91. [PubMed] [Google Scholar]
- 12. Previc FH. Vestibular loss as a contributor to Alzheimer's disease. Med Hypotheses. 2013;80(4):360‐367. [DOI] [PubMed] [Google Scholar]
- 13. Smith PF. The vestibular system and cognition. Curr Opin Neurol. 2017;30(1):84‐89. [DOI] [PubMed] [Google Scholar]
- 14. Bigelow RT, Agrawal Y. Vestibular involvement in cognition: visuospatial ability, attention, executive function, and memory. J Vestib Res Equilib Orientat. 2015;25(2):73‐89. [DOI] [PubMed] [Google Scholar]
- 15. Redfern MS, Talkowski ME, Jennings JR, Furman JM. Cognitive influences in postural control of patients with unilateral vestibular loss. Gait Posture. 2004;19(2):105‐114. [DOI] [PubMed] [Google Scholar]
- 16. Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S13‐S21. [PubMed] [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 18. Kalbe E, Kessler J, Calabrese P, et al. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004;19(2):136‐143. [DOI] [PubMed] [Google Scholar]
- 19. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271‐276. [Google Scholar]
- 20. Royall DR, Mulroy AR, Chiodo LK, Polk MJ. Clock drawing is sensitive to executive control: a comparison of six methods. J Gerontol B Psychol Sci Soc Sci. 1999;54(5):P328‐P333. [DOI] [PubMed] [Google Scholar]
- 21. Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64(5):588‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40(12):1221‐1226. [DOI] [PubMed] [Google Scholar]
- 23. Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A Clinical Test Arch Neurol. 1978;35(6):364‐367. [DOI] [PubMed] [Google Scholar]
- 24. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry J Ment Sci. 1979;134:382‐389. [DOI] [PubMed] [Google Scholar]
- 25. Tschan R, Wiltink J, Best C, et al. Validation of the German version of the vertigo symptom scale (VSS) in patients with organic or somatoform dizziness and healthy controls. J Neurol. 2008;255(8):1168‐1175. [DOI] [PubMed] [Google Scholar]
- 26. Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424‐427. [DOI] [PubMed] [Google Scholar]
- 27. Hüfner K, Hamilton DA, Kalla R, et al. Spatial memory and hippocampal volume in humans with unilateral vestibular deafferentation. Hippocampus. 2007;17(6):471‐485. [DOI] [PubMed] [Google Scholar]
- 28. Péruch P, Lopez C, Redon‐Zouiteni C, et al. Vestibular information is necessary for maintaining metric properties of representational space: evidence from mental imagery. Neuropsychologia. 2011;49(11):3136‐3144. [DOI] [PubMed] [Google Scholar]
- 29. Risey J, Briner W. Dyscalculia in patients with vertigo. J Vestib Res Equilib Orientat. 1990. 1991;1(1):31‐37. [PubMed] [Google Scholar]
- 30. Bigelow RT, Semenov YR, Trevino C, et al. Association between visuospatial ability and vestibular function in the Baltimore longitudinal study of aging. J Am Geriatr Soc. 2015;63(9):1837‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harun A, Oh ES, Bigelow RT, Studenski S, Agrawal Y. Vestibular impairment in dementia. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2016;37(8):1137‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamil RJ, Bilgel M, Wong DF, Resnick SM, Agrawal Y. Vestibular function and Beta‐amyloid deposition in the Baltimore longitudinal study of aging. Front Aging Neurosci. 2018;10:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev. 2015;13(1):CD005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindell E, Kollén L, Finizia C. Dizziness symptoms, balance confidence, and vestibular function in older women reporting dizziness and unsteadiness. Otol Neurotol off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. 2022;43(4):e482‐e488. [DOI] [PubMed] [Google Scholar]
- 35. Obermann M, Bock E, Sabev N, et al. Long‐term outcome of vertigo and dizziness associated disorders following treatment in specialized tertiary care: the Dizziness and Vertigo Registry (DiVeR) Study. J Neurol. 2015;262(9):2083‐2091. [DOI] [PubMed] [Google Scholar]
- 36. Prell T, Finn S, Axer H. How healthcare utilization due to dizziness and vertigo differs between older and younger adults. Front Med. 2022;9:852187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lacroix E, Deggouj N, Edwards MG, Van Cutsem J, Van Puyvelde M, Pattyn N. The cognitive‐vestibular compensation hypothesis: how cognitive impairments might be the cost of coping with compensation. Front Hum Neurosci. 2021;15:732974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


