Abstract
Anti-Muellerian hormone (AMH) is produced by the granulosa cells of ovarian follicles. It serves as a sensitive laboratory parameter for assessing ovarian reserve. A reduced ovarian reserve has been observed in patients with various autoimmune diseases. To compare serum levels of AMH as a surrogate parameter for ovarian reserve in female patients with systemic sclerosis compared to healthy controls and thereby assess fertility. In this single centre study from the University Hospital Tuebingen, Germany, we used serum samples to determine concentrations of AMH via an electrochemiluminescence immunoassay. We analysed 30 premenopausal female patients with systemic sclerosis and 30 age-matched healthy controls from 18 to 40 years. Patients who had received cyclophosphamide were excluded from this study. AMH levels were significantly reduced in patients with systemic sclerosis (955 ng/l versus 1.940 ng/L, p < 0.01). Interestingly, in contrast to healthy controls, we observed no significant correlation between age and AMH levels in patients. For women diagnosed with systemic sclerosis, especially at a younger age, regular assessment of AMH levels should be considered to improve guidance with regard to optimal pregnancy timepoint, fertility preservation and treatment options.
Keywords: Systemic sclerosis, Autoimmune diseases, Fertility, Anti-Muellerian hormone
Introduction
Anti-Muellerian hormone (AMH) is s a glycoprotein produced by the granulosa cells of ovarian follicles. It serves as a sensitive laboratory parameter for assessing ovarian reserve, which refers to the number and quality of a woman’s remaining eggs. Furthermore, AMH levels correlate with the antral follicle count and are relatively stable throughout the menstrual cycle. Therefore, it is an important factor in estimating individual reproductive potential.
In the context of fertility, AMH levels provide valuable insights into a woman's reproductive lifespan and her response to fertility treatments. Low AMH levels suggest diminished ovarian reserve, often associated with reduced fertility and poorer outcomes in assisted reproductive technologies such as in vitro fertilization (IVF) [1, 2].
In severe cases of systemic sclerosis (SSc) with a poor prognosis, cyclophosphamide (CYC) and high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) are used for treatment. The latest guidelines of the European League Against Rheumatism (EULAR) for SSc have also included ASCT in the recommendations for the treatment of severe lung and skin involvement [3]. Nevertheless, intensive therapies also have a downside, and the gonadal toxicity of CYC, for example, has long been known in patients with haematologic and rheumatic diseases [4, 5]. Furthermore, disease activity and other immunosuppressants might also adversely affect ovarian function. Higher rates of nulliparity and a lower number of children in patients with SSc have been described [6, 7]. Assessing AMH levels in these patients can help healthcare providers tailor fertility preservation strategies and optimize reproductive health management.
As many rheumatic diseases affect women at child bearing age, reproductive health is an important quality of life issue and has been addressed in the past [8–11]. Our group demonstrated a reduced ovarian reserve in patients with systemic lupus erythematosus, rheumatoid arthritis, spondyloarthritis, and behçet's disease [10, 12]. This has also been shown for patients with SSc, especially when they were exposed to CYC in the past [13]. Its impact on reproduction and fertility however has not been elucidated.
Aim of this study was to investigate ovarian reserve by AMH in female SSc patients at a reproductive age who never received CYC and compare them to an age matched healthy control.
Patients and methods
Patients and healthy controls
This monocentric study addresses ovarian reserve measured by AMH levels and clinical fertility parameters of female patients with SSc who were treated at the Department of Internal Medicine II, Tuebingen University Hospital, Germany from 2011 to 2023 in collaboration with the Department of Obstetrics and Gynaecology. Thirty-three consecutive patients were screened, and 30 Patients were eligible fulfilling all of the following inclusion and exclusion criteria: fulfilling the ACR-EULAR classification criteria for SSc [14], age between 18 and 40 years, no prior radiation therapy, no prior CYC therapy, no prior chemotherapy due to malignant disease, and normal renal function by creatinine clearance. Medical record data extraction included ongoing immunosuppressants and other disease related medication: prednisone, mycophenolate mofetil (MMF), azathioprine (AZA), methotrexate (MTX), sildenafil, bosentan and for typical anti-nuclear antibodies (ANA): anti-topoisomerase I (Scl-70), anti-centromere (ACA), anti-RNA Polymerase III and anti-PM/Scl. All patients answered a questionnaire on lifestyle and fertility and reproductive data.
Age-matched healthy controls (HC) were recruited in the Department of Obstetrics and Gynaecology.
Main outcome variable
This study investigates the ovarian reserve from using the estimation of AMH in female patients with SSc at a reproductive age who never received CYC.
Procedures
Serum for AMH was separated and stored at -20°C until used for assay. AMH was measured in duplicates by electrochemiluminescence immunoassay (ECLIA; Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocol. The test measures concentrations between 0.01 and 23 ng/ml. Depending on age, normal values range from 0.05 to 9.49 ng/ml for women. Values < 0.05 ng/ml are considered an indicator for limited ovarian reserve.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 10. Variables were compared between patients and controls using the Mann–Whitney test for AMH levels as this value did not show normal distribution in the Shapiro–Wilk test. p < 0.05 was considered statistically significant.
Ethical statement
The study was approved by the local ethics committee of the Eberhard-Karls-University Tuebingen (IRB 454/2023BO2) and all participants gave their written informed consent.
Results
Table 1 summarizes the cohort’s characteristics. Median age at time of AMH assessment was 32 years (min–max: 18–40), median disease duration was 28 months (min–max: 1–233), and n = 17 (56%) of the patients showed Scl70 positivity and n = 13 (43%) were diagnosed as dcSSc with a median mRSS of 16 (min–max: 0–40). Regarding the treatment for SSc at the time of AMH measurement: n = 9 (30%) of the patients were taking low-dose prednisone (i.e. ≤ 5mg prednisone per day), n = 8 (27%) MTX, n = 6 (20%) MMF, n = 3 (10%) AZA and n = 5 (17%) bosentan.
Table 1.
Patients ‘ characteristics
| Characteristic | All SSc patients |
|---|---|
| Fertility parameters | |
| Age at measurement (years), median (min–max) | 32 (18–40) |
| Number of children, median (min–max) | 0 (0–2) |
| Age at first child (years), median (min–max) | 30 (21–35) |
| Timing of menarche, (years), median (min–max) | 13 (9–20) |
|
Regularity in menstruation Regular cycle (no.[%]) Unregular cycle (no.[%]) Amenorrhea (no.[%]) |
23 (77) 6 (20) 1 (3) |
| Unfulfilled child wish (no.[%]) | 7 (23) |
| Misscarriage (no.[%]) | 3 (10) |
|
Smoking Ongoing (no.[%]) Never (no.[%]) |
2 (7) 28 (93) |
| Disease characteristics | |
| Disease duration (months), median (min–max) | 28 (1–233) |
|
Disease manifestation (no.[%]) dcSSc lcSSc Overlap |
13 (50) 15 (43) 2 (6) |
|
Autoantibody (no.[%]) None Scl70 ACA Other |
6 (20) 17 (57) 5 (17) 4 (6) |
| mRSS, median (min–max) | 7 (0–40) |
| Lung involvement (no.[%]) | 18 (60) |
| Heart involvement (no.[%]) | 2 (7) |
| Treatment at measurement* | |
| Mycophenolate mofetil | 6 (20) |
| Prednisone | 9 (30) |
| Azathioprine | 3 (10) |
| Methotrexate | 8 (27) |
| Sildenafil | 1 (3) |
| Bosentan | 5 (17) |
ACA, anti-centromere antibody, dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic sclerosis; max, maximum; min, minimum; mRSS, modified Rodnan skin score; no., number; Scl70, anti-topoisomerase II; SSc, systemic sclerosis
Fertility and reproduction
Median age at menarche was 13 years (min–max: 9–20), most (n = 23; 77%) of the patients reported a regular cycle and about one third (n = 10; 33%) of the patients had children (median 1; min–max: 0–2) with the median age of 29.5 years (min–max: 21–35) at the birth of the first child. Three patients (10%) reported of a prior miscarriage and n = 7 (23%) of a child wish but involuntary non-conception.
AMH levels in patients with systemic sclerosis compared to healthy controls
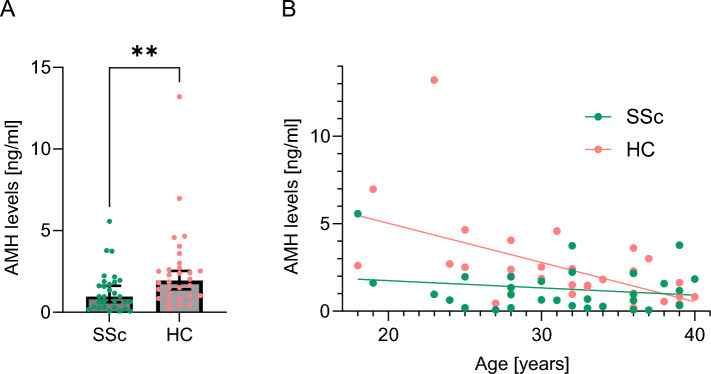
Overall AMH levels were significantly reduced in patients with SSc compared to age-matched HC (median value of 0.955 ng/l [min 0.065 to max 5,57] versus 1.940 ng/l [0.203–13.21], p < 0.01) (Fig. 1A). None of the patients showed AMH levels < 0.05 ng/ml.
Fig. 1.
Serum levels of Anti-Muellerian hormone (AMH) in 30 patients with systemic sclerosis (SSc) and 30 age-matched healthy controls (HC). A AMH levels were significantly lower in patients with SSc compared to HC (0.955 ng/l versus 1.940 ng/L, p < 0.01). B AMH concentration showed an inverse correlation between age at the timepoint of AMH measurement in HC (R2 = 0.282, p < 0.01) but not in SSc (R2 = 0.038, p = 0.34)
Comparative analysis of AMH levels and clinical parameters
As expected, AMH serum levels inversely correlated with age in HC (R2 = 0.282, p < 0.01), this was however not the case for women with SSc (R2 = 0.038, p = 0.34).
To determine, whether immunosuppressants might influence AMH levels, we stratified patients for different medications (patients who were exposed to CYC were excluded in this study): MMF (n = 6), glucocorticoids (GC; n = 9) as high as prednisone ≤ 5mg per day, AZA (n = 3), MTX (n = 8), sildenafil (n = 1) and bosentan (n = 5). Patients who received MMF, GC or bosentan showed significantly lower levels to HC (Fig. 2A). When considering only patients with or without a specific medication, only patients with or without bosentan showed a significant difference (Fig. 2B, non-significant data not shown).
Fig. 2.
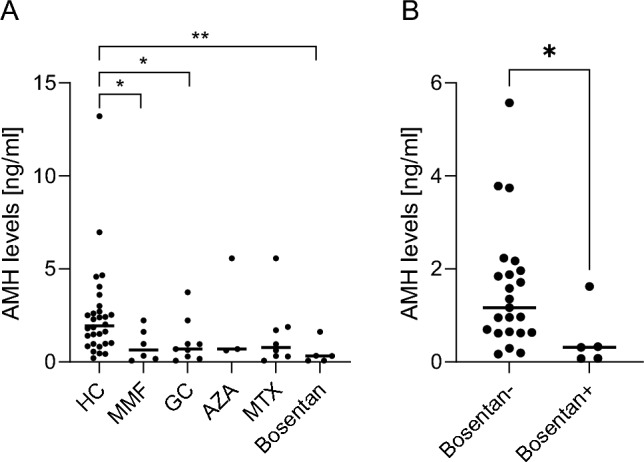
A/B Serum levels of Anti-Muellerian hormone (AMH) in patients with systemic sclerosis receiving different therapies (myophenolate mofetil [MMF], glucocorticoids [GC] as high as prednisone ≤ 5mg per day, azathioprine [AZA], and methotrexate [MTX]) compared to healthy controls (HC)) (2A). In addition patients with or without bosentan showed a significant difference (2B)
Patients who were not able to have children despite the desire to become pregnant for ≥ 12 months showed comparable levels of AMH (0.96 ng/ml versus 0.95 ng/ml, difference not significant).
Discussion
Different authors have shown that women with rheumatic diseases often exhibit reduced AMH levels compared to healthy controls [10, 12, 15, 16], but few studies have been executed to assess fertility in patients with SSc. Our study demonstrates that also in premenopausal patients with SSc the serum AMH level is diminished compared to healthy controls. Lower AMH levels suggest a reduced ovarian reserve, which can affect fertility. We therefore conclude, that women with SSc may face challenges in conceiving and may require fertility preservation strategies. Similar results were obtained by Goloeva et al. [13], however they also included patients who had received CYC. Since CYC is known to impair fertility, the authors were unable to distinguish between the potential association of CYC or disease-related factors and reduced AMH levels. Our data therefore confirm a diminished ovarian reserve in patients with SSc even without the known negative impact of prior therapy with CYC.
Several factors might contribute to AMH reduction in patients with SSc: Chronic inflammation associated with SSc might negatively impact ovarian function and lead to lower AMH levels. Furthermore, fibrosis and vascular damage, the pathologic hallmarks of SSc, might also affect the ovarian tissue which could lead to impaired ovarian function and reduced AMH levels.
For women diagnosed with SSc, especially at a younger age, regular assessment of AMH levels and fertility preservation techniques such as egg or embryo freezing should be considered. Hormonal replacement therapy may be beneficial for managing symptoms of primary ovarian insufficiency and improving quality of life, though it must be carefully weighed against potential risks, especially in autoimmune conditions [17, 18].
Increased risk of miscarriage has been reported in some, but not all analyses of patients with SSc [6, 19, 20]. In our analysis three patients (10%) reported of a prior miscarriage which is comparable to the general population [21]. However, we observed a high percentage of patients with child wish but involuntary non-conception (23%, n = 7) whereas the number of the general population is usually estimated to be low at about 5% [22, 23]. We did not observe a significant difference in between women with sustained or fulfilled child wish, suggesting that also other factors might influence the high number.
Our study has limitations, as this is a small single centre cohort and therefore generalizability might be limited. The small sample sizes can lead to a lack of statistical power, therefore interpretation of AMH levels in the different medication groups is only possible to a limited extent. More research is needed to confirm these results and understand the underlying mechanisms leading to reduced AMH levels in SSc. Therefore, also longitudinal follow-ups, investigating the role of immunosuppressive treatments and their impact on ovarian reserve are essential.
Reduced AMH levels in patients with systemic sclerosis highlight the intersection between autoimmune disease and reproductive health. Recognizing and addressing this issue is crucial for improving the overall well-being and quality of life of women with SSc. A proactive approach, including early diagnosis of the fertility status, e.g. by estimation by AMH levels will help us when counselling SSc patients especially with regard to (early) fertility preservation, such us social freezing, optimal timepoint for pregnancy and also with regard to therapeutic strategies by using more targeted therapies instead of CYC. This will mitigate the reproductive challenges faced by these patients. Further research will continue to elucidate the complex relationship between SSc and ovarian function, paving the way for better management strategies in the future.
Acknowledgements
The authors thank Margarita Deperschmidt for her unfailing technical support.
Funding
Open Access funding enabled and organized by Projekt DEAL. ACP was funded by the Clinician Scientist Program of the Medical Faculty, University of Tuebingen [grant number 477–0-0].
Declarations
Conflict of interests
Ann-Christin Pecher received honoraria by Novartis, UCB, Boehringer Ingelheim. Joerg Henes received grant support for SSc studies by Miltenyi and NEOVI and honoraria for lectures by ABBVIE, Astra-Zeneca, BMS, GSK, Novartis, UCB, Boehringer Ingelheim; Pfizer, Johnson & Johnson; SOBI. All other authors stated no conflict of interest.
Ethical standards
The study was approved by the local ethics committee of the Eberhard-Karls-University Tuebingen (IRB 454/2023BO2) and all participants gave their written informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nelson SM, Shaw M, Ewing BJ, McLean K, Vechery A, Briggs SF (2024) Antimullerian hormone levels are associated with time to pregnancy in a prospective cohort study of 3,150 women. Fertil Steril. 10.1016/j.fertnstert.2024.06.024 [DOI] [PubMed] [Google Scholar]
- 2.Volovsky M, Seifer DB (2024) Current status of ovarian and endometrial biomarkers in predicting ART outcomes. J Clin Med 13:13. 10.3390/jcm13133739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galdo FD, Lescoat A, Conaghan PG, Ananyeva LP, Balbir-Gurman A, Bertoldo E, Boyadzhieva V, Castellví I, Colic J, Denton CP, Distler O, Aoufy KE, Emmel J, Farrington S, Gabrielli A, Galetti I, Hoffmann-Vold AM, Kowal-Bielecka O, Cerinic MM, Müller-Ladner U, Ostojic P, Rednic S, Santiago T, Suliman YA, Tarasova A, Vonk M, Allanore Y (2023) OP0234 2023 update of eular recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 82(Suppl 1):154–155. 10.1136/annrheumdis-2023-eular.138335961761 [Google Scholar]
- 4.Wetzels JF (2004) Cyclophosphamide-induced gonadal toxicity: a treatment dilemma in patients with lupus nephritis? Neth J Med 62(10):347–352 [PubMed] [Google Scholar]
- 5.Leiper A, Houwing M, Davies EG, Rao K, Burns S, Morris E, Laven J, van der Kooi AL, van den Heuvel EM, Nussey S (2020) Anti-Mullerian hormone and Inhibin B after stem cell transplant in childhood: a comparison of myeloablative, reduced intensity and treosulfan-based chemotherapy regimens. Bone Marrow Transplant 55(10):1985–1995. 10.1038/s41409-020-0866-9 [DOI] [PubMed] [Google Scholar]
- 6.Steen VD, Medsger TA Jr (1999) Fertility and pregnancy outcome in women with systemic sclerosis. Arthritis Rheum 42(4):763–768. 10.1002/1529-0131(199904)42:4%3c763::AID-ANR21%3e3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 7.Bernatsky S, Hudson M, Pope J, Vinet E, Markland J, Robinson D, Jones N, Docherty P, Abu-Hakima M, Leclercq S, Dunne J, Smith D, Mathieu JP, Khalidi N, Sutton E, Baron M, Canadian Scleroderma Research G (2008) Assessment of reproductive history in systemic sclerosis. Arthritis Rheum 59(11):1661–1664. 10.1002/art.24198 [DOI] [PubMed] [Google Scholar]
- 8.Ostensen M (2017) Sexual and reproductive health in rheumatic disease. Nat Rev Rheumatol 13(8):485–493. 10.1038/nrrheum.2017.102 [DOI] [PubMed] [Google Scholar]
- 9.Grygiel-Gorniak B, Masiero E, Nevaneeth BC, Jojy MM (2023) Rheumatic diseases in reproductive age-the possibilities and the risks. Reprod Sci 30(1):111–123. 10.1007/s43032-022-00901-6 [DOI] [PubMed] [Google Scholar]
- 10.Henes M, Froeschlin J, Taran FA, Brucker S, Rall KK, Xenitidis T, Igney-Oertel A, Lawrenz B, Henes JC (2015) Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic diseases: impact of rheumatoid arthritis, Behcet’s disease and spondyloarthritis on anti-Mullerian hormone levels. Rheumatology (Oxford) 54(9):1709–1712. 10.1093/rheumatology/kev124 [DOI] [PubMed] [Google Scholar]
- 11.Wallenius M, Skomsvoll JF, Irgens LM, Salvesen KA, Nordvag BY, Koldingsnes W, Mikkelsen K, Kaufmann C, Kvien TK (2011) Fertility in women with chronic inflammatory arthritides. Rheumatology (Oxford) 50(6):1162–1167. 10.1093/rheumatology/keq458 [DOI] [PubMed] [Google Scholar]
- 12.Lawrenz B, Henes J, Henes M, Neunhoeffer E, Schmalzing M, Fehm T, Kitter I (2011) Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: evaluation by using anti-Muellerian hormone. Lupus 20(11):1193–1197. 10.1177/0961203311409272 [DOI] [PubMed] [Google Scholar]
- 13.Goloeva R, Alekberova Z, Cherkasova M, Koneva O, Garzanova L, Lila A (2022) AB0673 ovarian reserve in systemic sclerosis, systemic lupus erythematosus and Behcet’s disease. Ann Rheum Dis 81(Suppl 1):1464–1464. 10.1136/annrheumdis-2022-eular.2009 [Google Scholar]
- 14.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr, Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Muller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Csuka ME, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE (2013) 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 65(11):2737–2747. 10.1002/art.38098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mont’Alverne AR, Yamakami LY, Goncalves CR, Baracat EC, Bonfa E, Silva CA (2015) Diminished ovarian reserve in Behcet’s disease patients. Clin Rheumatol 34(1):179–183. 10.1007/s10067-014-2680-5 [DOI] [PubMed] [Google Scholar]
- 16.Luo W, Mao P, Zhang L, Chen X, Yang Z (2020) Assessment of ovarian reserve by serum anti-Mullerian hormone in patients with systemic lupus erythematosus: a meta-analysis. Ann Palliat Med 9(2):207–215 [DOI] [PubMed] [Google Scholar]
- 17.Holroyd CR, Edwards CJ (2009) The effects of hormone replacement therapy on autoimmune disease: rheumatoid arthritis and systemic lupus erythematosus. Climacteric 12(5):378–386. 10.1080/13697130903025449 [DOI] [PubMed] [Google Scholar]
- 18.Cutolo M, Lahita RG (2005) Estrogens and arthritis. Rheum Dis Clin North Am 31(1):19–27. 10.1016/j.rdc.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Silman AJ, Black C (1988) Increased incidence of spontaneous abortion and infertility in women with scleroderma before disease onset: a controlled study. Ann Rheum Dis 47(6):441–444. 10.1136/ard.47.6.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taraborelli M, Ramoni V, Brucato A, Airo P, Bajocchi G, Bellisai F, Biasi D, Blagojevic J, Canti V, Caporali R, Caramaschi P, Chiarolanza I, Codullo V, Cozzi F, Cuomo G, Cutolo M, De Santis M, De Vita S, Di Poi E, Doria A, Faggioli P, Favaro M, Ferraccioli G, Ferri C, Foti R, Gerosa A, Gerosa M, Giacuzzo S, Giani L, Giuggioli D, Imazio M, Iudici M, Iuliano A, Leonardi R, Limonta M, Lojacono A, Lubatti C, Matucci-Cerinic M, Mazzone A, Meroni M, Meroni PL, Mosca M, Motta M, Muscara M, Nava S, Padovan M, Pagani G, Paolazzi G, Peccatori S, Ravagnani V, Riccieri V, Rosato E, Rovere-Querini P, Salsano F, Santaniello A, Scorza R, Tani C, Valentini G, Valesini G, Vanoli M, Vigone B, Zeni S, Tincani A, Investigators I (2012) Brief report: successful pregnancies but a higher risk of preterm births in patients with systemic sclerosis: an Italian multicenter study. Arthritis Rheum 64(6):1970–1977. 10.1002/art.34350 [DOI] [PubMed] [Google Scholar]
- 21.Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst R, Lucas ES, McCoy RC, Anderson R, Daher S, Regan L, Al-Memar M, Bourne T, MacIntyre DA, Rai R, Christiansen OB, Sugiura-Ogasawara M, Odendaal J, Devall AJ, Bennett PR, Petrou S, Coomarasamy A (2021) Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 397(10285):1658–1667. 10.1016/S0140-6736(21)00682-6 [DOI] [PubMed] [Google Scholar]
- 22.Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G (2005) Definition and prevalence of subfertility and infertility. Hum Reprod 20(5):1144–1147. 10.1093/humrep/deh870 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt L (2006) Infertility and assisted reproduction in Denmark. Epidemiology and psychosocial consequences. Dan Med Bull 53(4):390–417 [PubMed] [Google Scholar]