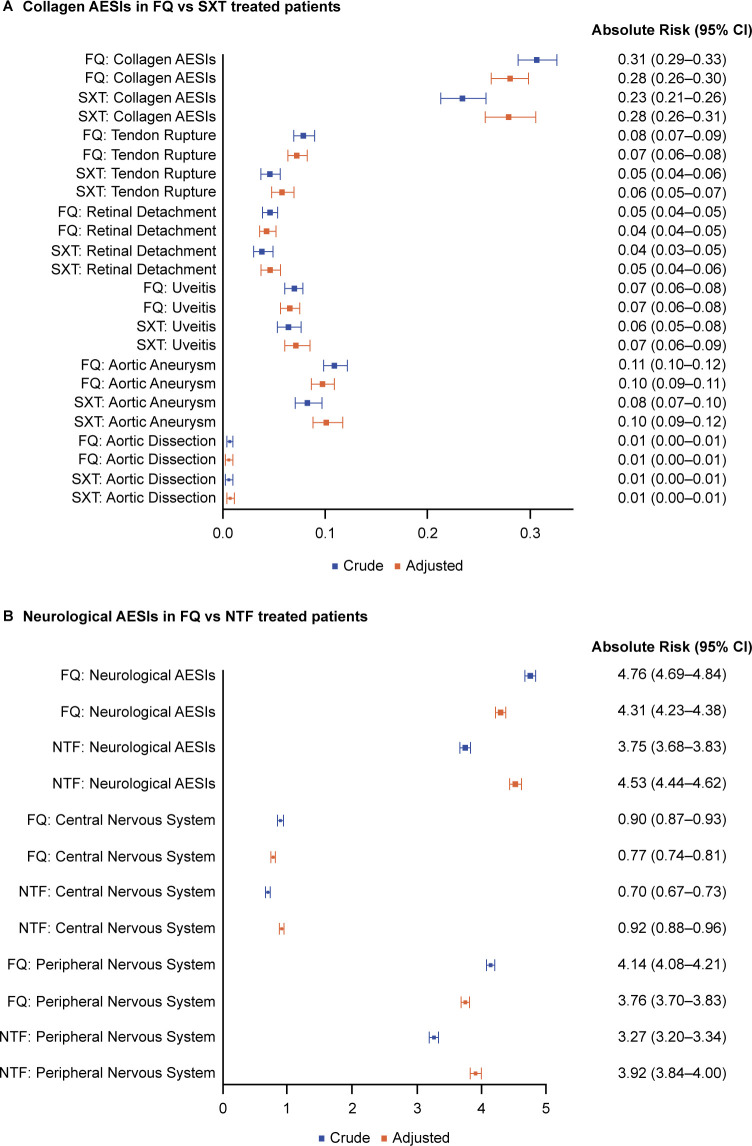
Fig 4.
Forest plot of crude and adjusted absolute risks of (A) collagen and (B) neurological AESIs. Collagen AESIs comprised tendon rupture, aortic aneurysm with or without dissection, retinal detachment, uveitis, and a composite category for all collagen AESIs. Neurological AESIs comprised CNS AESIs (seizures/convulsions, intracranial hypertension, psychosis/delirium, and altered mental status/encephalopathy), PNS AESIs (muscle weakness, paresthesia/sensory disturbance [tingling, numbness, burning pain, and allodynia], gait dysfunction, and peripheral neuropathy), and a composite category for all CNS and PNS AESIs. AESIs, adverse events of special interest; CNS, central nervous system; FQ, fluoroquinolone; NTF, nitrofurantoin; PNS, peripheral nervous system; sIPTW, inverse probability of treatment weighting; SXT, trimethoprim/sulfamethoxazole.