Abstract
The position-selective C–H bond activation of arenes has long been a challenging topic. Herein, we report an expedient ruthenium-electrocatalyzed site-selective ortho-C–H phosphorylation of arenes driven by electrochemical hydrogen evolution reaction (HER), avoiding stoichiometric amounts of chemical redox-waste products. This strategy paved the way to achieve unprecedented ruthenaelectro-catalyzed para-C–H phosphorylation with excellent levels of site-selectivity. This electrocatalytic approach was characterized by an ample substrate scope with a broad range of arenes containing N-heterocycles, as well as several aryl/alkylphosphine oxides were well tolerated. Moreover, late-stage C–H phosphorylation of medicinal relevant drugs could also be achieved. DFT mechanistic studies provided support for an unusual ruthenium(iii/iv/ii) regime for the ortho-C–H phosphorylation.
Sustainable ruthenaelectro-catalyzed position-selective ortho- and para-C–H phosphorylation of arenes by HER enabled late-stage functionalization, and the catalyst's mode of action was unraveled through experimental and computational studies.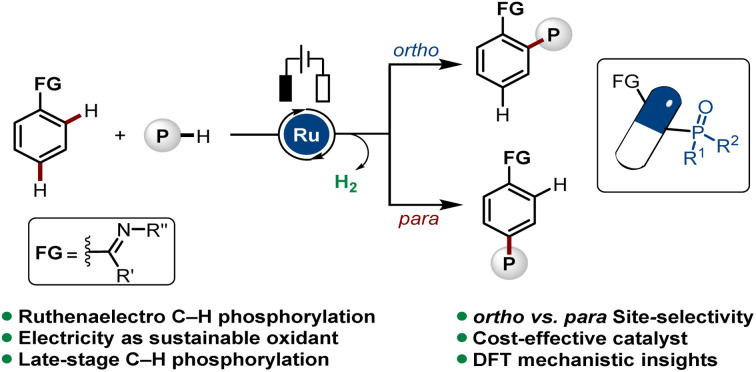
Introduction
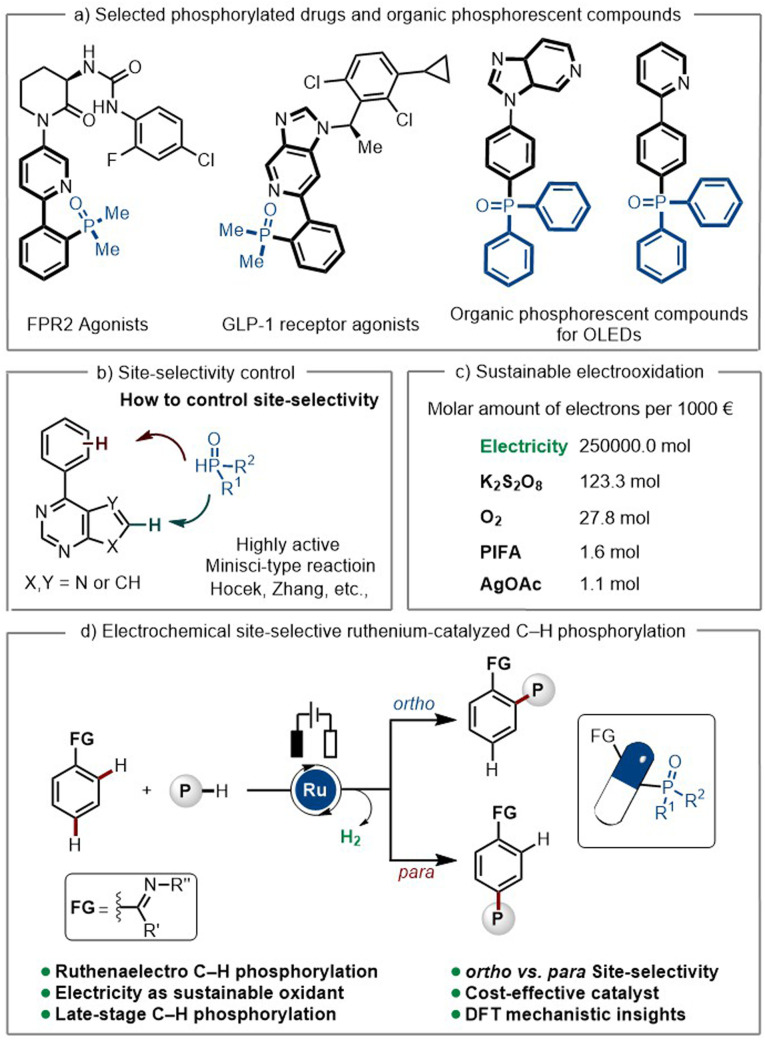
Organophosphorus compounds have been widely employed in organic synthesis,1 medicinal chemistry,2 material sciences,3 as well as prominent ligands4 in catalytic reactions. The presence of a phosphoryl group in target molecules has often been reported to enhance their physical-chemical properties, such as their hydrophilicity, thereby improving their solubility and tolerance in biological systems.5 Compounds bearing phosphine oxide motifs have revealed potential in decreasing inflammation, reducing blood sugar, and even anti-HIV activity.6 Additionally, phosphorylated heteroarenes have been reported as the one of the main components in phosphorescent OLEDs, due to their excellent luminescence properties (Scheme 1a).7 In this context, the development of novel and efficient strategies for the construction of aromatic C–P bonds poses great significance.
Scheme 1. Electrochemical ruthenium-catalyzed site-selective C–H phosphorylation.
Transition metal-catalyzed C–H activation has emerged as a powerful tool in modern synthesis.8 Direct C–H phosphorylation of arenes has proven to be challenging due to the strong coordination ability of the phosphine reagents, which often leads to catalyst deactivation. To overcome this drawback, distinct strategies have been developed. These involve the slow addition of the phosphine reagents,9 the use of masked phosphine reagents, which slowly release the active phosphine compounds10 or their sequential addition.11 However, these approaches involve the use of stoichiometric amounts of chemical oxidants, which strongly jeopardizes the sustainability of the overall approaches. On a different note, phosphine radical-based strategies for the synthesis of aryl phosphonyl compounds have been limited to electron-rich arenes, with poor position-selectivity.12 Furthermore, Minisci-type reactivity of nitrogen-containing heterocycles based on phosphine radicals is predominant,13 rendering phosphorylation at remote position difficult.
Ruthenium catalysis has surfaced as a uniquely versatile platform for proximal and distal bond functionalizations.14–16 Hence, we wondered whether position-selective ruthenium-catalyzed C–H phosphorylation would be viable in a position-selectivity-divergent manner by the judicious choice of the reaction conditions. The emergence of electrochemistry applied to organic synthesis has strongly revolutionized molecular synthesis by avoiding the use of chemical oxidants, leading to more sustainable and environmentally friendly synthetic routes,17 such as in transition metal-catalyzed C–H activation.18–23
Electrochemically24 driven ortho-C–H phosphorylation has solely been accomplished with expensive rhodium catalysts25 or through nickel catalysis, employing high-temperature conditions (110 °C, DG = 8-aminoquinoline).26 In sharp contrast, studies on metalla-electrocatalyzed C–H phosphorylations with versatile ruthenium catalysts have thus far proven elusive. Herein, we report a mild, electrochemically driven and cost-effective position-selective ruthenaelectro-catalyzed C–H phosphorylation with controlled position-switch from ortho to para. Moreover, phosphonyl units could be successfully introduced into relevant pharmaceutical compounds via late-stage C–H phosphorylation to access structurally diverse active compounds in a single step.
Results and discussion
Reaction optimization of ortho-C–H phosphorylation
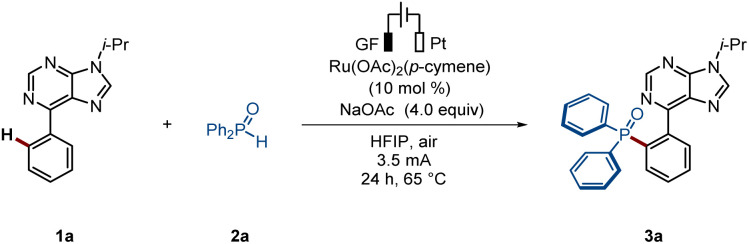
We initiated our studies by exploring the envisioned ruthenium-electrocatalyzed ortho-C–H phosphorylation of arene 1a and diphenylphosphine oxide 2a using a graphite felt (GF) and a platinum (Pt) electrode as anode and cathode materials, respectively, in an undivided cell setup (Table 1). The use of [Ru(OAc)2(p-cymene)] as the catalyst, in the presence of HFIP as the solvent, led to the formation of the desired phosphorylated product 3a, with complete ortho-selectivity, in 73% isolated yield (entry 1). Several other solvents and bases were also considered, but significantly reduced efficacy was noted (entries 2–3). Furthermore, other transition metal catalysts, such as Co(OAc)2·4H2O, Cu(OAc)2 or Ni(DME)Cl2, gave unsatisfactory results (entry 4). Control experiments demonstrated that electricity is crucial for the C–H phosphorylation (entry 6).
Optimization of the ortho-C–H phosphorylation reaction conditionsa.
| ||
|---|---|---|
| Entry | Deviation from standard conditions | Yield of 3ab(%) |
| 1 | None | 73 |
| 2 | H2O/CH3CN/TFE as solvent | −/−/65 |
| 3 | Na2CO3/Na3PO4/NaOPiv as base | 62/66/53 |
| 4c | Co(OAc)2·4H2O/Cu(OAc)2/Ni(DME)Cl2 | Trace/0/0 |
| 5 | N2 instead of air | 72 |
| 6 | Without electricity | 0 |
Reaction conditions A: undivided cell, GF anode, Pt cathode, 1a (0.3 mmol), 2a (4.0 equiv.), [Ru(OAc)2(p-cymene)] (10 mol%), NaOAc (4.0 equiv.), HFIP: 1,1,1,3,3,3-hexafluoropropan-2-ol (3.0 mL), air, 65 °C, 3.5 mA, 24 h.
Isolated yields.
Co(OAc)2·4H2O (10 mol%)/Cu(OAc)2 (10 mol%)/Ni(DME)Cl2 (10 mol%).
Substrate scope investigation for the ortho-C–H phosphorylation
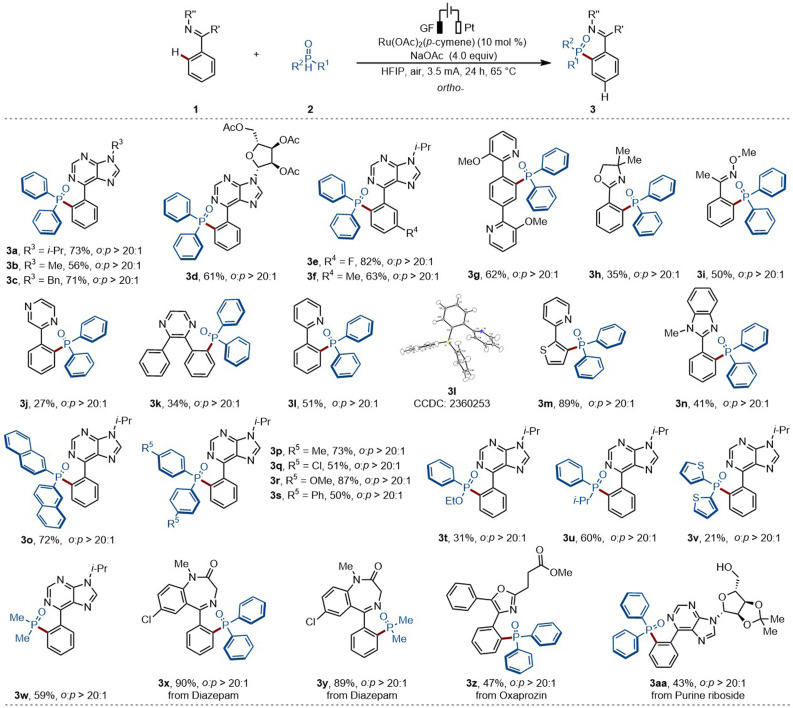
With the optimized reaction conditions in hand, we first investigated the viable scope of the ruthenium-electrocatalyzed ortho-C–H phosphorylation (Scheme 2). First, various N-substituted arylpurines were probed, including bio-relevant 6-phenylpurine nucleosides, efficiently affording the desired products in good yields (3a–3d). Additionally, both electron-withdrawing groups (e.g., –F) and electron-donating groups (e.g., –Me) on the arenes were well tolerated (3e–3f). Second, a bipyridine-substituted arene was also tolerated, delivering solely the mono-phosphorylation product (3g). Moreover, oxime derivatives selectively delivered the desired product (3i). Third, various types of arenes were explored, featuring a wide range of oxazolinyl, pyrazyl or pyridyl substituents (3j–3n). Afterwards, the scope of phosphorus coupling partners was further investigated. Fourth, diphenylphosphine oxides bearing electron-donating and electron-withdrawing groups were shown to be suitable coupling partners leading to the formation of the expected products 3o–3s in good yields. Other pentavalent phosphine oxides, comprised of phenylalkyl phosphine oxides, dithienyl phosphine oxides, and commonly used dimethyl phosphine oxides, in phosphorylated drugs, have been demonstrated to be successful coupling partners (3t–3w).
Scheme 2. Scope of the ortho-selective C–H phosphorylation.
Phosphorylation motifs can confide biological activity to drug molecules.5 In this context, the direct installation of phosphorylation units into target molecules, accounts for a more sustainable access to structural diversity, facilitating the expansion of the chemical space. Hence, we wondered whether our strategy could be exploited for late-stage C–H phosphorylations. To our delight, diazepam - a therapeutic drug used for acute tension and anxiety states - proved to be an amenable substrate, delivering the desired products 3x–3y in high yields. Additionally, oxaprozin and 6-phenylpurine riboside, delivered the desired products (3z–3aa) with excellent levels of position-selectivity.
Mechanistic studies of ortho-C–H phosphorylation
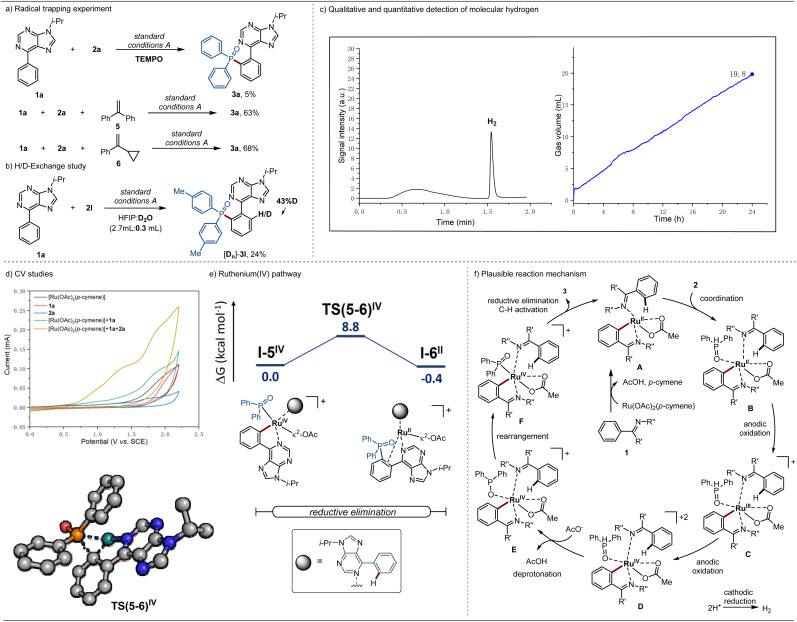
To gain insights into the reaction mechanism, a series of control experiments were conducted. The addition of equimolar amounts of TEMPO resulted in the inhibition of the ortho-C–H phosphorylation. However, given that TEMPO is a good reducing agent,27 such observations do not conclusively support the formation of a P-centered radical. Therefore, additional mechanistic experiments were conducted in the presence of representative radical scavengers, including 1,2-diphenylethylene and vinylcyclopropane 6, which is known to undergo ring opening in the presence of radial species. As a result, the ortho-phosphorylation product 3a was obtained in comparable isolated yields of 63% and 68%, respectively. These findings provide support for a non-radical pathway (Scheme 3a). Next, the addition of D2O as co-solvent resulted in a H/D scrambling at the ortho-position, suggesting a reversible ortho-C–H activation (Scheme 3b).
Scheme 3. (a) Radical trapping experiment. (b) H/D-Exchange study. (c) Headspace GC analysis after catalysis (left side) and measurement of gas evolution during catalysis (right side). (d) Cyclic voltammetry studies. (e) Relative Gibbs free energies (ΔG338.15) are given in kcal mol−1 for the ruthenium-catalyzed ortho-C–H phosphorylation reductive elimination step at the PBE0-D4/def2-TZVPP-SMD(HFIP)//PBE0-D3BJ/def2-SVP level of theory. In the computed transition state structure, non-relevant hydrogens were omitted for clarity. (f) Plausible reaction mechanism for the ruthenaelectro-catalyzed ortho-C–H phosphorylation.27.
In order to gain insights into the cathodic process, headspace gas chromatography was used and the formation of molecular hydrogen was probed. Thus, we monitored and quantified the formation of molecular hydrogen during the electrocatalytic reaction, which was determined to be 19.8 mL by the end of the electrocatalysis, translating into a faradaic efficiency of 59% (Scheme 3c and ESI Fig. S2, S3†). The result confirmed the hydrogen evolution reaction (HER) as the primary cathodic process, highlighting the unique potential of ruthenium electrocatalysis as a sustainable technology for organic synthesis.
Additionally, cyclic voltammetric (CV) experiments were conducted to assess the redox potential of the substrates as well as the catalyst (Scheme 3d). For the ortho-C–H phosphorylation, the mixture of phenyl-9H-purine (1a), diphenylphosphine oxide (2a), and the catalyst [Ru(OAc)2(p-cymene)] exhibited oxidation peaks at Ep/2 = 0.95 V and Ep/2 = 1.26 V vs. SCE. These findings are indicative of a ruthenacycle generated after C–H activation being coordinated by SPO 2a.
The catalyst mode of action for the ruthenium-electrocatalyzed ortho-C–H phosphorylation was further investigated through DFT calculations at the PBE0-D4/def2-TZVPP-SMD(HFIP)//PBE0-D3BJ/def2-SVP level of theory (Scheme 3e, Fig. S8 and S9, in the ESI†).28 Upon phosphine coordination two single electron oxidation steps take place, leading to the formation of the ruthenium(iv) intermediate I–1IV (Fig. S8†). Such is consistent with the CV studies, where two oxidation peaks were observed in the presence of phosphine. Subsequently, a facile phosphine deprotonation takes place (TS(2-3)) with an energy barrier of 6.8 kcal mol−1 giving rise to intermediate I-3, which after rearrangement originates a more exergonic intermediate I-5. The latter undergoes reductive elimination through transition state TS(5-6) with an energy barrier of 8.8 kcal mol−1. Additionally, an alternative pathway for reductive elimination under ruthenium(iii) was also investigated (Fig. S9†). The latter has proven to be energetically disfavored, not only by the prohibitive calculated barrier of 40.3 kcal mol−1 but also by the formation of an endergonic ruthenium(i) intermediate I-6I. Such observations provide support for a ruthenium(III/IV/II) regime.
Based on our experimental and computational mechanistic studies, a plausible reaction mechanism is depicted in Scheme 3f. The ortho-phosphorylation commences with the ortho-C–H activation, forming the ruthenacycle complex A. Subsequently, ligand exchange occurs, leading to a more easily oxidized intermediate B, which after two single electron oxidation steps originates the ruthenium(iv) intermediate D. Then, deprotonation gives rise to intermediate E, which rearranges to form intermediate F. Finally, F undergoes reductive elimination to yield the desired product 3 and to regenerate the active ruthenium(ii) catalyst A.
Reaction optimization of para-C–H phosphorylation
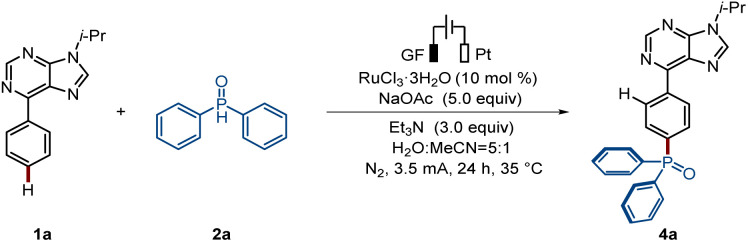
Next, we focused our attention on the envisioned switch towards ruthenaelectro-catalyzed para-C–H phosphorylation. We began our studies with arene 1a and diphenylphosphine oxide 2a using graphite felt (GF) and platinum (Pt) electrodes as anode and cathode materials, respectively, in an undivided cell setup (Table 2). When RuCl3·3H2O was used as a catalyst in the presence of MeCN as solvent a minor amount of para-C–H phosphorylated product was obtained. Upon further experimentation, the para-phosphorylated product 4a was selectively obtained in an isolated yield of 82% in the presence of RuCl3·3H2O, triethylamine, and a solvent mixture of MeCN and water (Entry 1). Notably, only unconverted substrate 1a was accounted for in the mass balance here, with no side product, from possible phosphorylation at the purine C–H bond, being detected. Other tested solvents and bases did not improve the efficacy of the reaction (entries 3–4). Moreover, the electricity was shown to be essential for the catalysis to take place (entry 6).
Optimization of the para-C–H phosphorylation reaction conditionsa.
| ||
|---|---|---|
| Entry | Deviation from standard conditions | Yield of 4ab (%) |
| 1 | None | 82 |
| 2 | Under air | 66 |
| 3 | H2O/CH3CN/TFE as solvent | 25/12/— |
| 4 | Na2CO3/Na3PO4/NaOPiv as base | 78/55/23 |
| 5c | PPh3/2,2′-bipyridine as ligand | 55/42 |
| 6 | Without electricity | 0 |
Reaction conditions B: undivided cell, GF anode, Pt cathode, 1a (0.3 mmol), 2a (4.0 equiv.), [RuCl3·3H2O] (10 mol%), NaOAc (5.0 equiv.), Et3N (3.0 equiv.), MeCN : H2O = (0.5 : 2.5 mL), N2, 35 °C, 3.5 mA, 24 h.
Isolated yields.
PPh3 (10 mol%), 2,2′-bipyridine (10 mol%).
Substrate scope investigation for the para-C–H phosphorylation
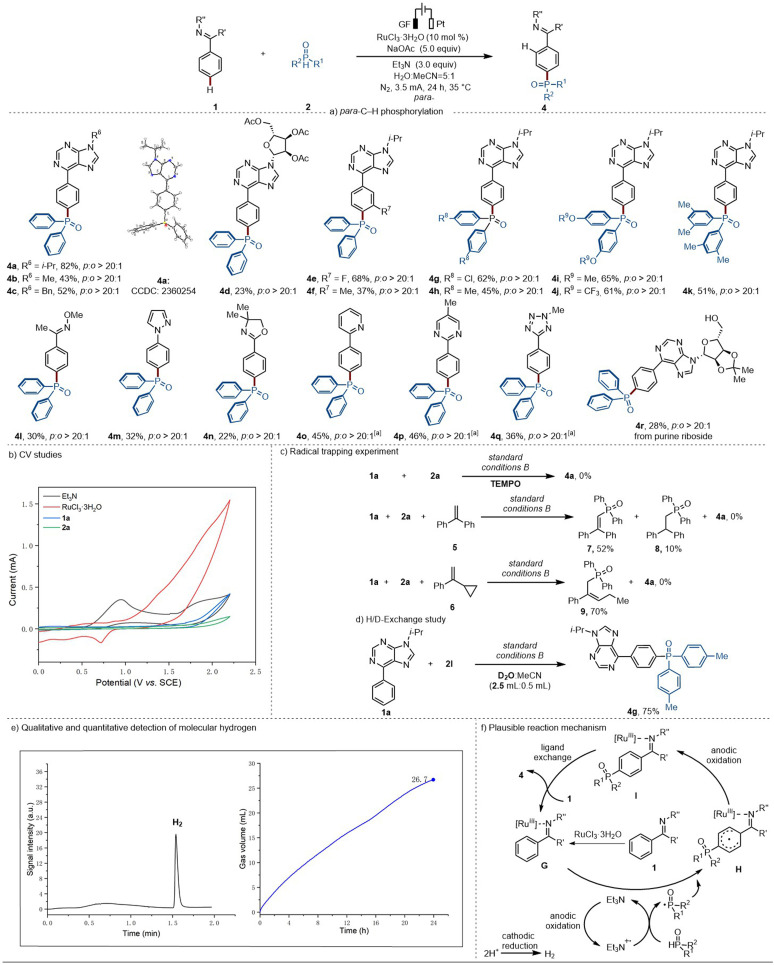
With the optimized reaction conditions in hand, we directed our attention to the viable substrate scope of the ruthenium-electrocatalyzed para-C–H phosphorylation (Scheme 4a). Several N-substituted 6-phenylpurines were explored, enabling the formation of the corresponding desired products in good yields with exclusive para-selectivity (4a–4d). Furthermore, both electron-withdrawing groups (–F) and electron-donating (–Me) on the arenes were well tolerated (4e–4f). Diphenylphosphine oxide compounds bearing different substituents (–Cl, –Me, –OMe, –OCF3) were also well tolerated (4g–4k). Noticeably, the 1-phenylpyrazole yielded the expected single para-C–H phosphorylation product, with no phosphorylation occurring at the pyrazole ring (4m). Arenes bearing different substituents, such as pyrimidyl, pyridyl, or tetrazolyl groups, demonstrated higher efficacy, in the absence of triethylamine, with excellent para-selectivity (4o–4q). Moreover, our approach could be successfully applied to the late-stage C–H phosphorylation of 6-phenylpurine riboside, delivering the desired product (4r) with excellent position-selectivity.
Scheme 4. (a) Scope of the ruthenium-electrocatalyzed para-selective C–H phosphorylation, ano Et3N. (b) Cyclic Voltammograms. (c) Radical trapping experiment. Yield ratio calculated by phosphorus NMR from a mixture of 7 and 8. (d) H/D-exchange study. (e) Headspace GC analysis after catalysis (left side) and measurement of gas evolution during catalysis (right side). (f) Plausible reaction mechanism involved in the ruthenaelectro-catalyzed of para-C–H phosphorylation.
Mechanistic studies for the para-C–H phosphorylation
To gain mechanistic insights into the ruthenium-electrocatalyzed para-C–H phosphorylation, cyclic voltammetry (CV) experiments were conducted to access the redox potential of the substrates as well as the catalyst (Scheme 4b). The Et3N features an oxidation peak at Ep/2 = 0.91 V vs. SCE, which is much lower than the one obtained for 1a, 2a, and RuCl3·3H2O, indicating that Et3N is more susceptible to be oxidized.
Additionally, a series of control experiments were performed. The addition of TEMPO under otherwise identical reaction conditions inhibited the para-C–H phosphorylation. Moreover, upon the addition of 1,2-diphenylethylene, the corresponding radical intermediates 7 and 8 were trapped. When vinylcyclopropane 6 was added, the compound resulting from the ring opening (9) could be isolated in 70% yield. These findings provide strong support for the involvement of a phosphorus-centered radical in the para-phosphorylation (Scheme 4c). However, when D2O was added to the para-phosphorylation reaction, no H/D-scrambling was observed, suggesting that an ortho-C–H cycloruthenation is not relevant for the para-reaction pathway (Scheme 4d).
Additionally, we monitored and quantified the formation of molecular hydrogen during the electrocatalytic reaction, which was determined to be 26.7 mL by the end of the reaction time, translating into a faradaic efficiency of 68% (Scheme 4e and ESI Fig. S4, S5†). This provides support for the hydrogen evolution reaction (HER) to be the primary cathodic process.
Based on our experimental mechanistic studies, a plausible reaction mechanism is depicted in Scheme 4f. For the para-phosphorylation pathway, the nitrogen-containing heteroarene first coordinates to the ruthenium(iii) catalyst to form intermediate G. Then, Et3N·+ may react with H-phosphonate or H-phosphine oxide to give rise to a phosphorus-centered radical. Subsequently, the electrophilic phosphine radical will attack at the para-position of the arene via a charge transfer-directed approach,29 followed by oxidative aromatization to generate the para-phosphorylated product. In the meanwhile, at the cathode, protons are reduced to generate molecular hydrogen by HER.
Conclusions
In conclusion, we have devised a position-selectivity switch for electrochemical ruthenium-catalyzed C–H phosphorylations enabled by hydrogen evolution reaction (HER). Thereby, we achieved selective ortho- and even para-C–H phosphorylation. The robustness of the ruthena-electrocatalysis was reflected by a wide substrate scope including various sensitive electrophilic functional groups. Our strategy thereby enabled challenging late-stage phosphorylations of biorelevant pharmaceuticals. Experimental and computational mechanistic studies provided strong support for an unusual ruthenium(iii/iv/ii) manifold for the ruthenaelectro-catalyzed proximal phosphorylation.
Data availability
All data associated with this study are available in the article and ESI.†
Author contributions
Conceptualization, L. A.; methodology, X.-Y. G.; investigation, X.-Y. G.; DFT calculation, J. C. A. O.; cyclic voltammetry studies, S. L. H.; headspace GC analysis and measurement, S .T.; HRMS studies, T. v. M.; writing – original Draft, X.-Y. G., B.-S. Z. and J. C. A. O.; writing – review & editing, X.-Y. G., J. C. A. O., and S. C.; funding acquisition, L. A.; resources, L. A.; supervision, L. A.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support from the ERC Advanced grant no. 101021358, the DFG (Gottfried Wilhelm Leibniz award to L. A., SPP2363). We thank Dr Christopher Golz (University of Göttingen) for the assistance with the X-ray diffraction analysis,30 Dr Holm Frauendorf for HRMS analysis and Dr Michael John for NMR spectroscopy.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06219a
Notes and references
- (a) Demmer C. S. Krogsgaard-Larsen N. Bunch L. Review on Modern Advances of Chemical Methods for the Introduction of a Phosphonic Acid Group. Chem. Rev. 2011;111:7981–8006. doi: 10.1021/cr2002646. [DOI] [PubMed] [Google Scholar]; (b) Zhu Y.-Y. Zhang T. Zhou L. Yang S.-D. Concise synthesis of N-phosphorylated amides through three-component reactions. Green Chem. 2021;23:9417–9421. doi: 10.1039/D1GC03065E. [DOI] [Google Scholar]
- Baguley T. D. Xu H.-C. Chatterjee M. Nairn A. C. Lombroso P. J. Ellman J. A. Substrate-Based Fragment Identification for the Development of Selective, Nonpeptidic Inhibitors of Striatal-Enriched Protein Tyrosine Phosphatase. J. Med. Chem. 2013;56:7636–7650. doi: 10.1021/jm401037h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon K. J. Perry H. P. Clearfield A. Conventional and Unconventional Metal–Organic Frameworks Based on Phosphonate Ligands: MOFs and UMOFs. Chem. Rev. 2012;112:1034–1054. doi: 10.1021/cr2002257. [DOI] [PubMed] [Google Scholar]
- (a) Holz J. Jiao H. Gandelman M. Börner A. About the Inversion Barriers of P-Chirogenic Triaryl-Substituted Phosphanes. Eur. J. Org Chem. 2018:2984–2994. doi: 10.1002/ejoc.201701734. [DOI] [Google Scholar]; (b) Ma Y.-N. Li S.-X. Yang S.-D. New Approaches for Biaryl-Based Phosphine Ligand Synthesis via P═O Directed C–H Functionalizations. Acc. Chem. Res. 2017;50:1480–1492. doi: 10.1021/acs.accounts.7b00167. [DOI] [PubMed] [Google Scholar]
- Tian C. A. Chiu C. C. Importance of Hydrophilic Groups on Modulating the Structural, Mechanical, and Interfacial Properties of Bilayers: A Comparative Molecular Dynamics Study of Phosphatidylcholine and Ion Pair Amphiphile Membranes. Int. J. Mol. Sci. 2018;19:1552–1571. doi: 10.3390/ijms19061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirude P. S., Chattopadhyay A. K. K., Ellen K. and Wurtz N. R., Biaryl dialkyl phosphine oxide Fpr2 agonists, WO2020257161A1, 2020
- (a) Zhao J. Feng Z. Zhong D. Yang X. Wu Y. Zhou G. Wu Z. Cyclometalated Platinum Complexes with Aggregation-Induced Phosphorescence Emission Behavior and Highly Efficient Electroluminescent Ability. Chem. Mater. 2018;30:929–946. doi: 10.1021/acs.chemmater.7b04708. [DOI] [Google Scholar]; (b) Sarada G. Maheshwaran A. Cho W. Lee T. Han S. H. Lee J. Y. Jin S.-H. Pure blue phosphorescence by new N-heterocyclic carbene-based Ir(III) complexes for organic light-emitting diode application. Dyes Pigm. 2018;150:1–8. doi: 10.1016/j.dyepig.2017.11.011. [DOI] [Google Scholar]
- (a) Docherty J. H. Lister T. M. McArthur G. Findlay M. T. Domingo-Legarda P. Kenyon J. Choudhary S. Larrosa I. Transition-Metal-Catalyzed C–H Bond Activation for the Formation of C–C Bonds in Complex Molecules. Chem. Rev. 2023;123:7692–7760. doi: 10.1021/acs.chemrev.2c00888. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rogge T. Kaplaneris N. Chatani N. Kim J. Chang S. Punji B. Schafer L. L. Musaev D. G. Wencel-Delord J. Roberts C. A. Sarpong R. Wilson Z. E. Brimble M. A. Johansson M. J. Ackermann L. C–H activation. Nat. Rev. Methods Primers. 2021;1:43. doi: 10.1038/s43586-021-00041-2. [DOI] [Google Scholar]; (c) Dutta U. Maiti S. Bhattacharya T. Maiti D. Arene diversification through distal C(sp2)−H functionalization. Science. 2021;372:eabd5992. doi: 10.1126/science.abd5992. [DOI] [PubMed] [Google Scholar]; (d) Karimov R. R. Hartwig J. F. Transition-Metal-Catalyzed Selective Functionalization of C(sp3)−H Bonds in Natural Products. Angew. Chem., Int. Ed. 2018;57:4234–4241. doi: 10.1002/anie.201710330. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhang Z. Tanaka K. Yu J.-Q. Remote site-selective C–H activation directed by a catalytic bifunctional template. Nature. 2017;543:538–542. doi: 10.1038/nature21418. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Davies H. M. L. Du Bois J. Yu J.-Q. C–H Functionalization in organic synthesis. Chem. Soc. Rev. 2011;40:1855–1856. doi: 10.1039/C1CS90010B. [DOI] [PubMed] [Google Scholar]; (g) Ackermann L. Carboxylate-Assisted Transition-Metal-Catalyzed C−H Bond Functionalizations: Mechanism and Scope. Chem. Rev. 2011;111:1315–1345. doi: 10.1021/cr100412j. [DOI] [PubMed] [Google Scholar]
- Feng C.-G. Ye M. Xiao K.-J. Li S. Yu J.-Q. Pd(II)-Catalyzed Phosphorylation of Aryl C–H Bonds. J. Am. Chem. Soc. 2013;135:9322–9325. doi: 10.1021/ja404526x. [DOI] [PubMed] [Google Scholar]
- (a) Min M. Kang D. Jung S. Hong S. Rhodium-Catalyzed Direct C–H Phosphorylation of (Hetero)arenes Suitable for Late-Stage Functionalization. Adv. Synth. Catal. 2016;358:1296–1301. doi: 10.1002/adsc.201600014. [DOI] [Google Scholar]; (b) Li C. Yano T. Ishida N. Murakami M. Pyridine-Directed Palladium-Catalyzed Phosphonation of C(sp2)-H Bonds. Angew. Chem., Int. Ed. 2013;52:9801–9804. doi: 10.1002/anie.201305202. [DOI] [PubMed] [Google Scholar]
- Wang S. Guo R. Wang G. Chen S.-Y. Yu X.-Q. Copper-catalyzed phosphorylation of sp2 C–H bonds. Chem. Commun. 2014;50:12718–12721. doi: 10.1039/C4CC06246A. [DOI] [PubMed] [Google Scholar]
- (a) Berger O. Montchamp J.-L. Manganese-Catalyzed and Mediated Synthesis of Arylphosphinates and Related Compounds. J. Org. Chem. 2019;84:9239–9256. doi: 10.1021/acs.joc.9b01239. [DOI] [PubMed] [Google Scholar]; (b) Niu L. Liu J. Yi H. Wang S. Liang X.-A. Singh A. K. Chiang C.-W. Lei A. Visible-Light-Induced External Oxidant-Free Oxidative Phosphonylation of C(sp2)–H Bonds. ACS Catal. 2017;7:7412–7416. doi: 10.1021/acscatal.7b02418. [DOI] [Google Scholar]; (c) Berger O. Montchamp J.-L. Manganese-Mediated Homolytic Aromatic Substitution with Phosphinylidenes. Chem. Rec. 2017;17:1203–1212. doi: 10.1002/tcr.201700021. [DOI] [PubMed] [Google Scholar]
- (a) Sabat N. Poštová Slavětínská L. Klepetářová B. Hocek M. C–H Phosphonation of Pyrrolopyrimidines: Synthesis of Substituted 7- and 9-Deazapurine-8-phosphonate Derivatives. J. Org. Chem. 2016;81:9507–9514. doi: 10.1021/acs.joc.6b01970. [DOI] [PubMed] [Google Scholar]; (b) Xiang C.-B. Bian Y.-J. Mao X.-R. Huang Z.-Z. Coupling Reactions of Heteroarenes with Phosphites under Silver Catalysis. J. Org. Chem. 2012;77:7706–7710. doi: 10.1021/jo301108g. [DOI] [PubMed] [Google Scholar]
- (a) Ogba O. M. Warner N. C. O'Leary D. J. Grubbs R. H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 2018;47:4510–4544. doi: 10.1039/C8CS00027A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ackermann L. Carboxylate-Assisted Ruthenium-Catalyzed Alkyne Annulations by C–H/Het–H Bond Functionalizations. Acc. Chem. Res. 2014;47:281–295. doi: 10.1021/ar3002798. [DOI] [PubMed] [Google Scholar]; (c) Arockiam P. B. Bruneau C. Dixneuf P. H. Ruthenium(II)-Catalyzed C–H Bond Activation and Functionalization. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. [DOI] [PubMed] [Google Scholar]; (d) Ackermann L. Vicente R. J. C. A. Ruthenium-catalyzed direct arylations through C–H bond cleavages. Top. Curr. Chem. 2010;292:211–229. doi: 10.1007/128_2009_9. [DOI] [PubMed] [Google Scholar]; (e) Noyori R. Hashiguchi S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997;30:97–102. doi: 10.1021/ar9502341. [DOI] [Google Scholar]
- (a) Wang G.-W. Wheatley M. Simonetti M. Cannas D. M. Larrosa I. Cyclometalated Ruthenium Catalyst Enables Ortho-Selective C–H Alkylation with Secondary Alkyl Bromides. Chem. 2020;6:1459–1468. doi: 10.1016/j.chempr.2020.04.006. [DOI] [Google Scholar]; (b) Fan W.-T. Li Y. Wang D. Ji S.-J. Zhao Y. Iron-Catalyzed Highly para-Selective Difluoromethylation of Arenes. J. Am. Chem. Soc. 2020;142:20524–20530. doi: 10.1021/jacs.0c09545. [DOI] [PubMed] [Google Scholar]; (c) Wang X.-G. Li Y. Liu H.-C. Zhang B.-S. Gou X.-Y. Wang Q. Ma J.-W. Liang Y.-M. Three-Component Ruthenium-Catalyzed Direct Meta-Selective C–H Activation of Arenes: A New Approach to the Alkylarylation of Alkenes. J. Am. Chem. Soc. 2019;141:13914–13922. doi: 10.1021/jacs.9b06608. [DOI] [PubMed] [Google Scholar]; (d) Simonetti M. Cannas D. M. Just-Baringo X. Vitorica-Yrezabal I. J. Larrosa I. Cyclometallated ruthenium catalyst enables late-stage directed arylation of pharmaceuticals. Nat. Chem. 2018;10:724–731. doi: 10.1038/s41557-018-0062-3. [DOI] [PubMed] [Google Scholar]; (e) Li G. Li D. Zhang J. Shi D.-Q. Zhao Y. Ligand-Enabled Regioselectivity in the Oxidative Cross-coupling of Arenes with Toluenes and Cycloalkanes Using Ruthenium Catalysts: Tuning the Site-Selectivity from the ortho to meta Positions. ACS Catal. 2017;7:4138–4143. doi: 10.1021/acscatal.7b01072. [DOI] [Google Scholar]; (f) Leitch J. A. Frost C. G. Ruthenium-catalysed σ-activation for remote meta-selective C–H functionalisation. Chem. Soc. Rev. 2017;46:7145–7153. doi: 10.1039/C7CS00496F. [DOI] [PubMed] [Google Scholar]; (g) Teskey C. J. Lui A. Y. W. Greaney M. F. Ruthenium-Catalyzed meta-Selective C-H Bromination. Angew. Chem., Int. Ed. 2015;54:11677–11680. doi: 10.1002/anie.201504390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Guillemard L. Ackermann L. Johansson M. J. Late-stage meta-C–H alkylation of pharmaceuticals to modulate biological properties and expedite molecular optimisation in a single step. Nat. Commun. 2024;15:3349–3358. doi: 10.1038/s41467-024-46697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Korvorapun K. Moselage M. Struwe J. Rogge T. Messinis A. M. Ackermann L. Regiodivergent C−H and Decarboxylative C−C Alkylation by Ruthenium Catalysis: ortho versus meta Position-Selectivity. Angew. Chem., Int. Ed. 2020;59:18795–18803. doi: 10.1002/anie.202007144. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gandeepan P. Koeller J. Korvorapun K. Mohr J. Ackermann L. Visible-Light-Enabled Ruthenium-Catalyzed meta-C−H Alkylation at Room Temperature. Angew. Chem., Int. Ed. 2019;58:9820–9825. doi: 10.1002/anie.201902258. [DOI] [PubMed] [Google Scholar]; (d) Li J. Korvorapun K. De Sarkar S. Rogge T. Burns D. J. Warratz S. Ackermann L. Ruthenium(II)-catalysed remote C–H alkylations as a versatile platform to meta-decorated arenes. Nat. Commun. 2017;8:15430. doi: 10.1038/ncomms15430. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hofmann N. Ackermann L. meta-Selective C–H Bond Alkylation with Secondary Alkyl Halides. J. Am. Chem. Soc. 2013;135:5877–5884. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]; (f) Ackermann L. Novák P. Vicente R. Hofmann N. Ruthenium-Catalyzed Regioselective Direct Alkylation of Arenes with Unactivated Alkyl Halides through C-H Bond Cleavage. Angew. Chem., Int. Ed. 2009;48:6045–6048. doi: 10.1002/anie.200902458. [DOI] [PubMed] [Google Scholar]
- (a) Gao M. Y. Gosmini C. J. I. J. O. C. Transition Metal-Catalyzed Electroreductive Cross-Couplings for C− C Bond Formation. Isr. J. Chem. 2024;64:e202300074. doi: 10.1002/ijch.202300074. [DOI] [Google Scholar]; (b) Ackermann L., Brown R. C. D., Enders P., Fang P., Folgueiras-Amador A. A., Francke R., Galczynski J., Gosmini C., Hodgson J. W., Hou Z. W., Huang H., Huang Z., Inagi S., Kuciński K., Kuriyama M., Lam K., Lambert T. H., Leech M. C., Lennox A. J. J., Lin Z., Little R. D., Massignan L., Mei T. S., Meyer T. H., Moeller K. D., Onomura O., Prudlik A., Ruan Z., Scheremetjew A., Schiltz P., Selt M., Villani E., Waldvogel S. R., Wang Z. H., Wu T., Xing Y. K., Xu H. C. and Yamamoto K., Electrochemistry in Organic Synthesis, 2022, https://dx.doi.org/10.1055/b000000126 [Google Scholar]; (c) Siu J. C. Fu N. Lin S. Catalyzing Electrosynthesis: A Homogeneous Electrocatalytic Approach to Reaction Discovery. Acc. Chem. Res. 2020;53:547–560. doi: 10.1021/acs.accounts.9b00529. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Meyer T. H. Choi I. Tian C. Ackermann L. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis? Chem. 2020;6:2484–2496. doi: 10.1016/j.chempr.2020.08.025. [DOI] [Google Scholar]; (e) Kingston C. Palkowitz M. D. Takahira Y. Vantourout J. C. Peters B. K. Kawamata Y. Baran P. S. A Survival Guide for the “Electro-curious”. Acc. Chem. Res. 2020;53:72–83. doi: 10.1021/acs.accounts.9b00539. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yuan Y. Lei A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution Reactions. Acc. Chem. Res. 2019;52:3309–3324. doi: 10.1021/acs.accounts.9b00512. [DOI] [PubMed] [Google Scholar]; (g) Xiong P. Xu H.-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019;52:3339–3350. doi: 10.1021/acs.accounts.9b00472. [DOI] [PubMed] [Google Scholar]; (h) Möhle S. Zirbes M. Rodrigo E. Gieshoff T. Wiebe A. Waldvogel S. R. Modern Electrochemical Aspects for the Synthesis of Value-Added Organic Products. Angew. Chem., Int. Ed. 2018;57:6018–6041. doi: 10.1002/anie.201712732. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yan M. Kawamata Y. Baran P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Alvarez E. M. Stewart G. Ullah M. Lalisse R. Gutierrez O. Malapit C. A. Site-Selective Electrochemical Arene C–H Amination. J. Am. Chem. Soc. 2024;146:3591–3597. doi: 10.1021/jacs.3c11506. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gao Y. Zhang B. He J. Baran P. S. Ni-Electrocatalytic Enantioselective Doubly Decarboxylative C(sp3)–C(sp3) Cross-Coupling. J. Am. Chem. Soc. 2023;145:11518–11523. doi: 10.1021/jacs.3c03337. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lou T. S.-B. Kawamata Y. Ewing T. Correa-Otero G. A. Collins M. R. Baran P. S. Scalable, Chemoselective Nickel Electrocatalytic Sulfinylation of Aryl Halides with SO2. Angew. Chem., Int. Ed. 2022;61:e202208080. doi: 10.1002/anie.202208080. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Saito M. Kawamata Y. Meanwell M. Navratil R. Chiodi D. Carlson E. Hu P. Chen L. Udyavara S. Kingston C. Tanwar M. Tyagi S. McKillican B. P. Gichinga M. G. Schmidt M. A. Eastgate M. D. Lamberto M. He C. Tang T. Malapit C. A. Sigman M. S. Minteer S. D. Neurock M. Baran P. S. N-Ammonium Ylide Mediators for Electrochemical C–H Oxidation. J. Am. Chem. Soc. 2021;143:7859–7867. doi: 10.1021/jacs.1c03780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Hou Z.-W. Yan H. Song J. Xu H.-C. Photoelectrocatalytic C–H amination of arenes. Green Chem. 2023;25:7959–7962. doi: 10.1039/D3GC02126B. [DOI] [Google Scholar]; (b) Chen N. Xu H.-C. Electrochemically Driven Radical Reactions: From Direct Electrolysis to Molecular Catalysis. Chem. Rec. 2021;21:2306–2319. doi: 10.1002/tcr.202100048. [DOI] [PubMed] [Google Scholar]; (c) Xu P. Chen P.-Y. Xu H.-C. Scalable Photoelectrochemical Dehydrogenative Cross-Coupling of Heteroarenes with Aliphatic C−H Bonds. Angew. Chem., Int. Ed. 2020;59:14275–14280. doi: 10.1002/anie.202005724. [DOI] [PubMed] [Google Scholar]; (d) Lai X.-L. Shu X.-M. Song J. Xu H.-C. Electrophotocatalytic Decarboxylative C−H Functionalization of Heteroarenes. Angew. Chem., Int. Ed. 2020;59:10626–10632. doi: 10.1002/anie.202002900. [DOI] [PubMed] [Google Scholar]; (e) Xu F. Li Y.-J. Huang C. Xu H.-C. Ruthenium-Catalyzed Electrochemical Dehydrogenative Alkyne Annulation. ACS Catal. 2018;8:3820–3824. doi: 10.1021/acscatal.8b00373. [DOI] [Google Scholar]
- (a) Yuan Y. Yang J. Lei A. Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals. Chem. Soc. Rev. 2021;50:10058–10086. doi: 10.1039/D1CS00150G. [DOI] [PubMed] [Google Scholar]; (b) Hu X. Nie L. Zhang G. Lei A. Electrochemical Oxidative [4+2] Annulation for the π-Extension of Unfunctionalized Heterobiaryl Compounds. Angew. Chem., Int. Ed. 2020;59:15238–15243. doi: 10.1002/anie.202003656. [DOI] [PubMed] [Google Scholar]; (c) Hu X. Zhang G. Nie L. Kong T. Lei A. Electrochemical oxidation induced intermolecular aromatic C-H imidation. Nat. Commun. 2019;10:5467–5476. doi: 10.1038/s41467-019-13524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hu X. Zhang G. Bu F. Nie L. Lei A. Electrochemical-Oxidation-Induced Site-Selective Intramolecular C(sp3)–H Amination. ACS Catal. 2018;8:9370–9375. doi: 10.1021/acscatal.8b02847. [DOI] [Google Scholar]
- (a) Xing Y.-K. Wang Z.-H. Fang P. Ma C. Mei T.-S. Divergent synthesis of aryl amines and dihydroquinazolinones via electrochemistry-enabled rhodium-catalyzed C–H functionalization. Sci. China:Chem. 2023;66:2863–2870. doi: 10.1007/s11426-023-1603-9. [DOI] [Google Scholar]; (b) Liu D. Liu Z.-R. Wang Z.-H. Ma C. Herbert S. Schirok H. Mei T.-S. Paired electrolysis-enabled nickel-catalyzed enantioselective reductive cross-coupling between α-chloroesters and aryl bromides. Nat. Commun. 2022;13:7318–7326. doi: 10.1038/s41467-022-35073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jiao K.-J. Xing Y.-K. Yang Q.-L. Qiu H. Mei T.-S. Site-Selective C–H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis. Acc. Chem. Res. 2020;53:300–310. doi: 10.1021/acs.accounts.9b00603. [DOI] [PubMed] [Google Scholar]
- (a) Michiyuki T. Maksso I. Ackermann L. Photo-Induced Ruthenium-Catalyzed C−H Arylation Polymerization at Ambient Temperature. Angew. Chem., Int. Ed. 2024:e202400845. doi: 10.1002/anie.202400845. [DOI] [PubMed] [Google Scholar]; (b) Lin Z. Oliveira J. C. A. Scheremetjew A. Ackermann L. Palladium-Catalyzed Electrooxidative Double C–H Arylation. J. Am. Chem. Soc. 2024;146:228–239. doi: 10.1021/jacs.3c08479. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang Y. Dana S. Long H. Xu Y. Li Y. Kaplaneris N. Ackermann L. Electrochemical Late-Stage Functionalization. Chem. Rev. 2023;123:11269–11335. doi: 10.1021/acs.chemrev.3c00158. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) von Münchow T. Dana S. Xu Y. Yuan B. Ackermann L. Enantioselective electrochemical cobalt-catalyzed aryl C–H activation reactions. Science. 2023;379:1036–1042. doi: 10.1126/science.adg2866. [DOI] [PubMed] [Google Scholar]; (e) Li Y. Dana S. Ackermann L. Recent advances in organic electrochemical functionalizations for specialty chemicals. Curr. Opin. Electrochem. 2023;40:101312. doi: 10.1016/j.coelec.2023.101312. [DOI] [Google Scholar]; (f) Wang Y. Simon H. Chen X. Lin Z. Chen S. Ackermann L. Distal Ruthenaelectro-Catalyzed meta-C−H Bromination with Aqueous HBr. Angew. Chem., Int. Ed. 2022;61:e202201595. doi: 10.1002/anie.202201595. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Tan X. Hou X. Rogge T. Ackermann L. Ruthenaelectro-Catalyzed Domino Three-Component Alkyne Annulation for Expedient Isoquinoline Assembly. Angew. Chem., Int. Ed. 2021;60:4619–4624. doi: 10.1002/anie.202014289. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ackermann L. Metalla-electrocatalyzed C–H Activation by Earth-Abundant 3d Metals and Beyond. Acc. Chem. Res. 2020;53:84–104. doi: 10.1021/acs.accounts.9b00510. [DOI] [PubMed] [Google Scholar]
- (a) Qi J. Xu J. Ang H. T. Wang B. Gupta N. K. Dubbaka S. R. O'Neill P. Mao X. Lum Y. Wu J. Electrophotochemical Synthesis Facilitated Trifluoromethylation of Arenes Using Trifluoroacetic Acid. J. Am. Chem. Soc. 2023;145:24965–24971. doi: 10.1021/jacs.3c10148. [DOI] [PubMed] [Google Scholar]; (b) Huang H. Steiniger K. A. Lambert T. H. Electrophotocatalysis: Combining Light and Electricity to Catalyze Reactions. J. Am. Chem. Soc. 2022;144:12567–12583. doi: 10.1021/jacs.2c01914. [DOI] [PubMed] [Google Scholar]; (c) Jin S. Kim J. Kim D. Park J.-W. Chang S. Electrolytic C–H Oxygenation via Oxidatively Induced Reductive Elimination in Rh Catalysis. ACS Catal. 2021;11:6590–6595. doi: 10.1021/acscatal.1c01670. [DOI] [Google Scholar]; (d) Luo M.-J. Hu M. Song R.-J. He D.-L. Li J.-H. Ruthenium(ii)-catalyzed electrooxidative [4+2] annulation of benzylic alcohols with internal alkynes: entry to isocoumarins. Chem. Commun. 2019;55:1124–1127. doi: 10.1039/C8CC08759H. [DOI] [PubMed] [Google Scholar]
- (a) Wang S. Xue Q. Guan Z. Ye Y. Lei A. ACS Catal. 2021;11:4295–4300. doi: 10.1021/acscatal.1c00549. [DOI] [Google Scholar]; (b) Kurimoto Y. Yamashita J. Mitsudo K. Sato E. Suga S. Org. Lett. 2021;23:3120–3124. doi: 10.1021/acs.orglett.1c00807. [DOI] [PubMed] [Google Scholar]; (c) Li K.-J. Jiang Y.-Y. Xu K. Zeng C.-C. Sun B.-G. Green Chem. 2019;21:4412–4421. doi: 10.1039/C9GC01474H. [DOI] [Google Scholar]
- Wu Z.-J. Su F. Lin W. Song J. Wen T.-B. Zhang H.-J. Xu H.-C. Scalable Rhodium(III)-Catalyzed Aryl C−H Phosphorylation Enabled by Anodic Oxidation Induced Reductive Elimination. Angew. Chem., Int. Ed. 2019;58:16770–16774. doi: 10.1002/anie.201909951. [DOI] [PubMed] [Google Scholar]
- Zhang S.-K. Del Vecchio A. Kuniyil R. Messinis A. M. Lin Z. Ackermann L. Electrocatalytic C–H phosphorylation through nickel(III/IV/II) catalysis. Chem. 2021;7:1379–1392. [Google Scholar]
- Qian X.-Y. Li S.-Q. Song J. Xu H.-C. TEMPO-Catalyzed Electrochemical C–H Thiolation: Synthesis of Benzothiazoles and Thiazolopyridines from Thioamides. ACS Catal. 2017;7:2730–2734. doi: 10.1021/acscatal.7b00426. [DOI] [Google Scholar]
- For detailed information see ESI.†
- Boursalian G. B. Ham W. S. Mazzotti A. R. Ritter T. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nat. Chem. 2016;8:810–815. doi: 10.1038/nchem.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For X-ray single crystal diffraction data: ; CCDC 2360253: Experimental Crystal Structure Determination, 2024, DOI: 10.5517/ccdc.csd.cc2k7162; ; CCDC 2360254: Experimental Crystal Structure Determination, 2024, DOI: 10.5517/ccdc.csd.cc2k7173.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are available in the article and ESI.†