Abstract
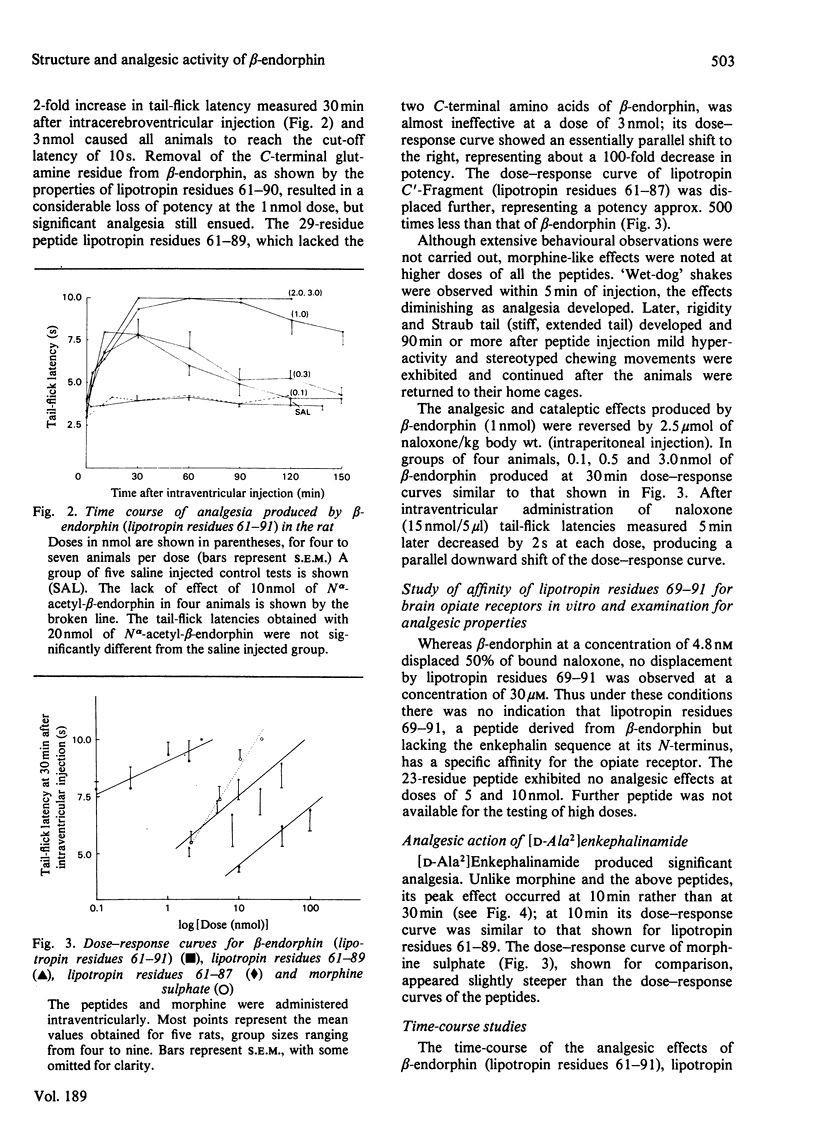
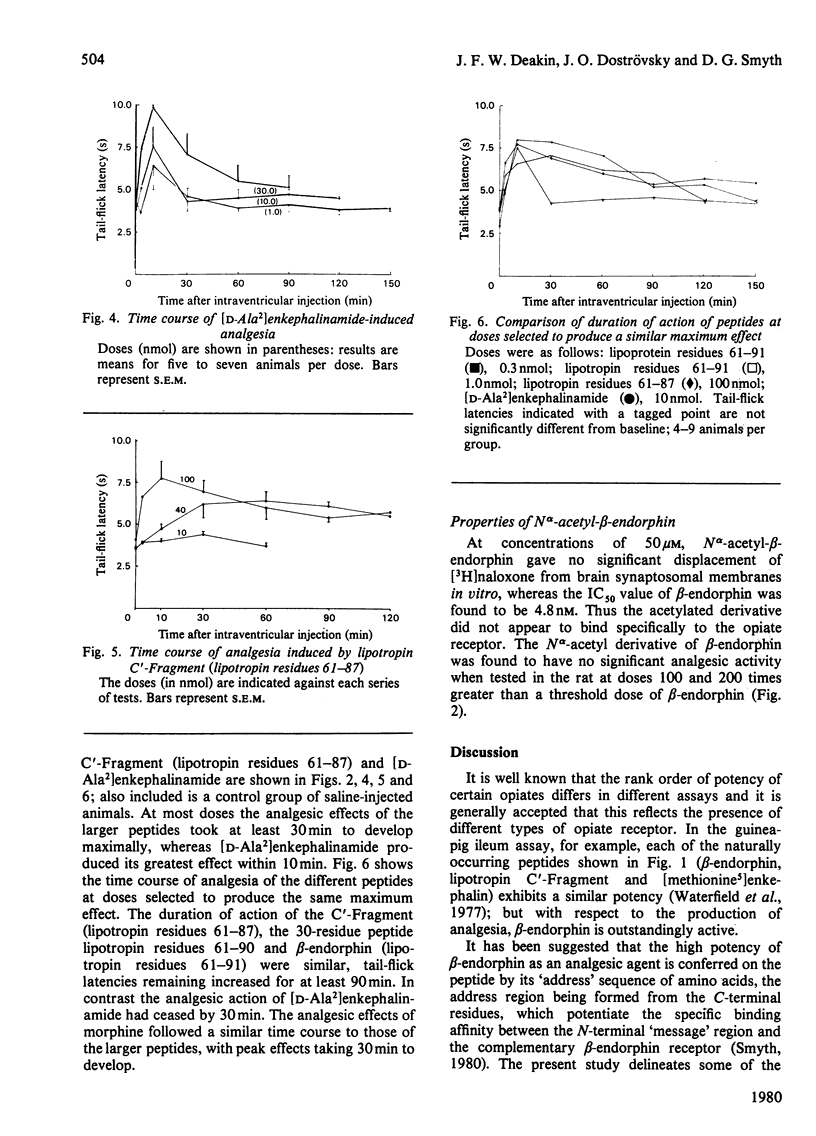
Removal of one, two and four amino-acid residues from the C-terminus of beta-endorphin ('lipotropin C-Fragment', lipotropin residues 61--91) led to the formation of peptides with progressively decreased analgesic potency; there was no change in the persistence of the analgesic effects. The four C-terminal residues are thus important for the activity of beta-endorphin, but not for the duration of action. Removal of eight amino-acid residues from the N-terminus provided a peptide that had no specific affinity for brain opiate receptors in vitro and was devoid of analgesic properties. The N-terminal sequence of beta-endorphin is therefore necessary for the production of analgesia, whereas the C-terminal residues confer potency. The N alpha-acetyl form of beta-endorphin had no specific affinity for brain opiate receptors in vitro and possessed no significant analgesic properties. Since lipotropin C'-Fragment (lipotropin residues 61--87) and the N alpha-acetyl derivative of beta-endorphin occur naturally in brain and pituitary and are only weakly active or inactive as opiates, it is suggested that proteolysis at the C-terminus and acetylation of the N-terminus of beta-endorphin may constitute physiological mechanisms for inactivation of this potent analgesic peptide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M., Evans C. J., Smyth D. G. Susceptibility of neuroactive peptides to aminopeptidase digestion is related to molecular size. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1211–1217. doi: 10.1016/0006-291x(79)91196-3. [DOI] [PubMed] [Google Scholar]
- Austen B. M., Smyth D. G. The NH2-terminus of C-fragment is resistant to the action of aminopeptidases. Biochem Biophys Res Commun. 1976 May 23;76(2):477–482. doi: 10.1016/0006-291x(77)90749-5. [DOI] [PubMed] [Google Scholar]
- Belluzzi J. D., Grant N., Garsky V., Sarantakis D., Wise C. D., Stein L. Analgesia induced in vivo by central administration of enkephalin in rat. Nature. 1976 Apr 15;260(5552):625–626. doi: 10.1038/260625a0. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J., Hulme E. C. C fragment of lipotropin has a high affinity for brain opiate receptors. Nature. 1976 Apr 29;260(5554):793–795. doi: 10.1038/260793a0. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G., Snell C. R., Deakin J. F., Wendlandt S. Comparison of the analgesic properties of lipotropin C-fragment and stabilized enkephalins in the rat. Biochem Biophys Res Commun. 1977 Jan 24;74(2):748–754. doi: 10.1016/0006-291x(77)90365-5. [DOI] [PubMed] [Google Scholar]
- Buscher H. H., Hill R. C., Römer D., Cardinaux F., Closse A., Hauser D., Pless J. Evidence for analgesic activity of enkephalin in the mouse. Nature. 1976 Jun 3;261(5559):423–425. doi: 10.1038/261423a0. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Smyth D. G. C-fragment of lipotropin--an endogenous potent analgesic peptide. Br J Pharmacol. 1977 Jul;60(3):445–453. doi: 10.1111/j.1476-5381.1977.tb07521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., Deakin J. F., Dostrovsky J. O., Smyth D. G. Analgesic activity of lipotropin C fragment depends on carboxyl terminal tetrapeptide. Nature. 1977 Sep 8;269(5624):167–168. doi: 10.1038/269167a0. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Loh H. H., Tseng L. F., Wei E., Li C. H. beta-endorphin is a potent analgesic agent. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2895–2898. doi: 10.1073/pnas.73.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Pert A., Chang J. K., Fong B. T. (D-Ala2)-Met-enkephalinamide: a potent, long-lasting synthetic pentapeptide analgesic. Science. 1976 Oct 15;194(4262):330–332. doi: 10.1126/science.968485. [DOI] [PubMed] [Google Scholar]
- Smyth D. G., Snell C. R., Massey D. E. Isolation of the C-fragment and C'-fragment of lipotropin from pig pituitary and C-fragment from brain. Biochem J. 1978 Oct 1;175(1):261–270. doi: 10.1042/bj1750261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. M., Berntson G. G., Sandman C. A., Coy D. H., Schally A. V., Kastin A. J. An analog of enkephalin having prolonged opiate-like effects in vivo. Science. 1977 Apr 1;196(4285):85–87. doi: 10.1126/science.190683. [DOI] [PubMed] [Google Scholar]
- Zakarian S., Smyth D. Distribution of active and inactive forms of endorphins in rat pituitary and brain. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5972–5976. doi: 10.1073/pnas.76.11.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree J. M., de Wied D., Bradbury A. F., Hulme E. C., Smyth D. C., Snell C. R. Induction of tolerance to the analgesic action of lipotropin in C-fragment. Nature. 1976 Dec 23;264(5588):792–794. doi: 10.1038/264792a0. [DOI] [PubMed] [Google Scholar]


