Figure 1 ∣. Hydroxyl-directed γ-oxygenation of secondary and tertiary alcohols and ketones.
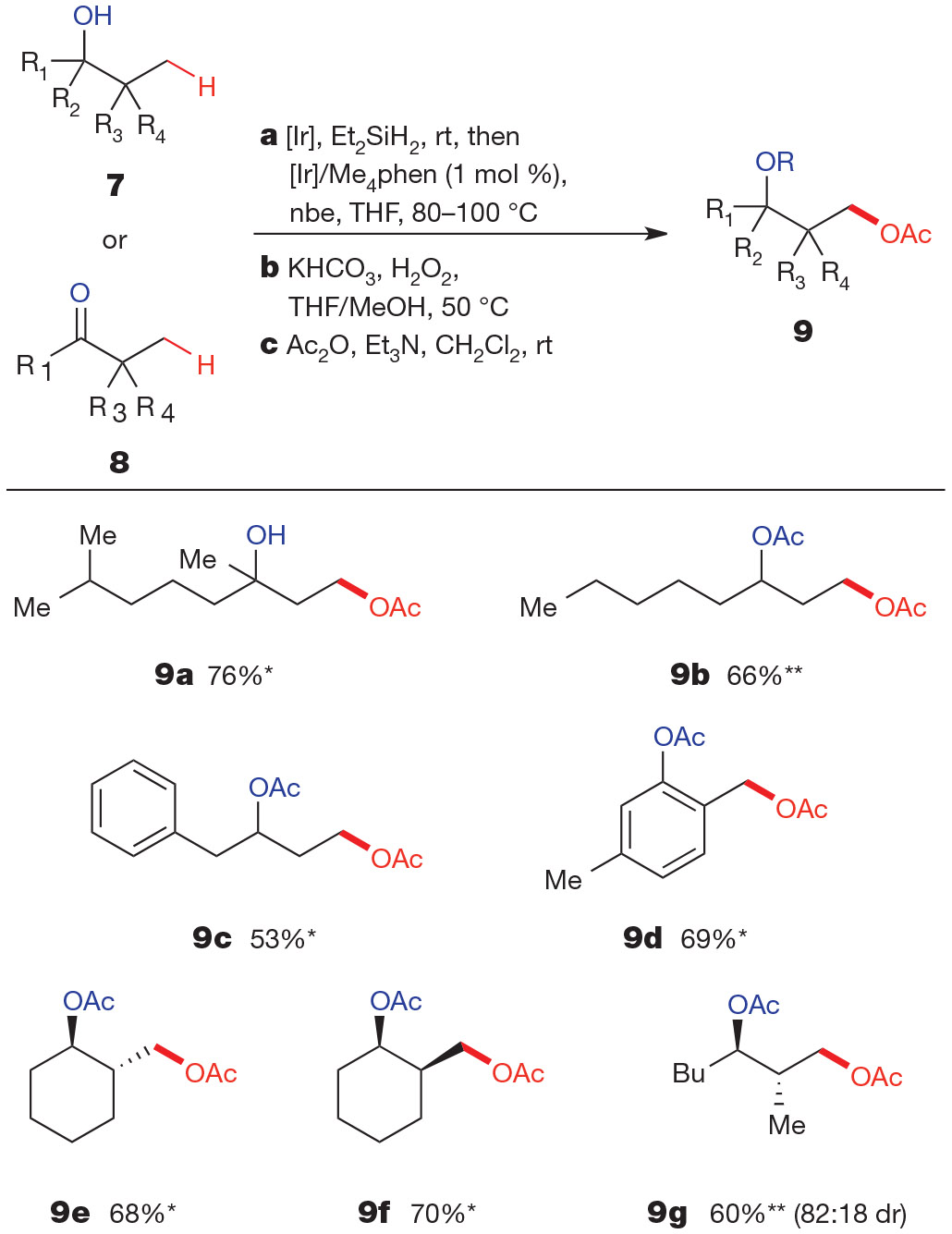
Overall isolated yields for reactions conducted on a 1.0-mmol scale following purification by silica-gel chromatography. Reagents and conditions: a, 7 (to give products marked *) or 8 (products marked **) (1.0 equiv.), Et2SiH2 (1.2 equiv.), [Ir(cod)OMe]2 (0.05 mol %), THF, room temperature (rt); removal of volatiles, then [Ir(cod)OMe]2 (0.5 mol %), Me4phen (1.2 mol %), nbe (1.2 equiv.), THF, 80–100 °C. b, KHCO3 (2.5 equiv.), 30% aqueous H2O2 (10 equiv.), THF/MeOH, 50 °C. c, Ac2O (1.5–3.0 equiv.), DMAP (0–0.05 equiv.), CH2Cl2/Et3N, room temperature. Ac2O, acetic anhydride; DMAP, 4-dimethylaminopyridine; Et3N, triethylamine. For full experimental details, see the Supplementary Information.
