Figure 4.
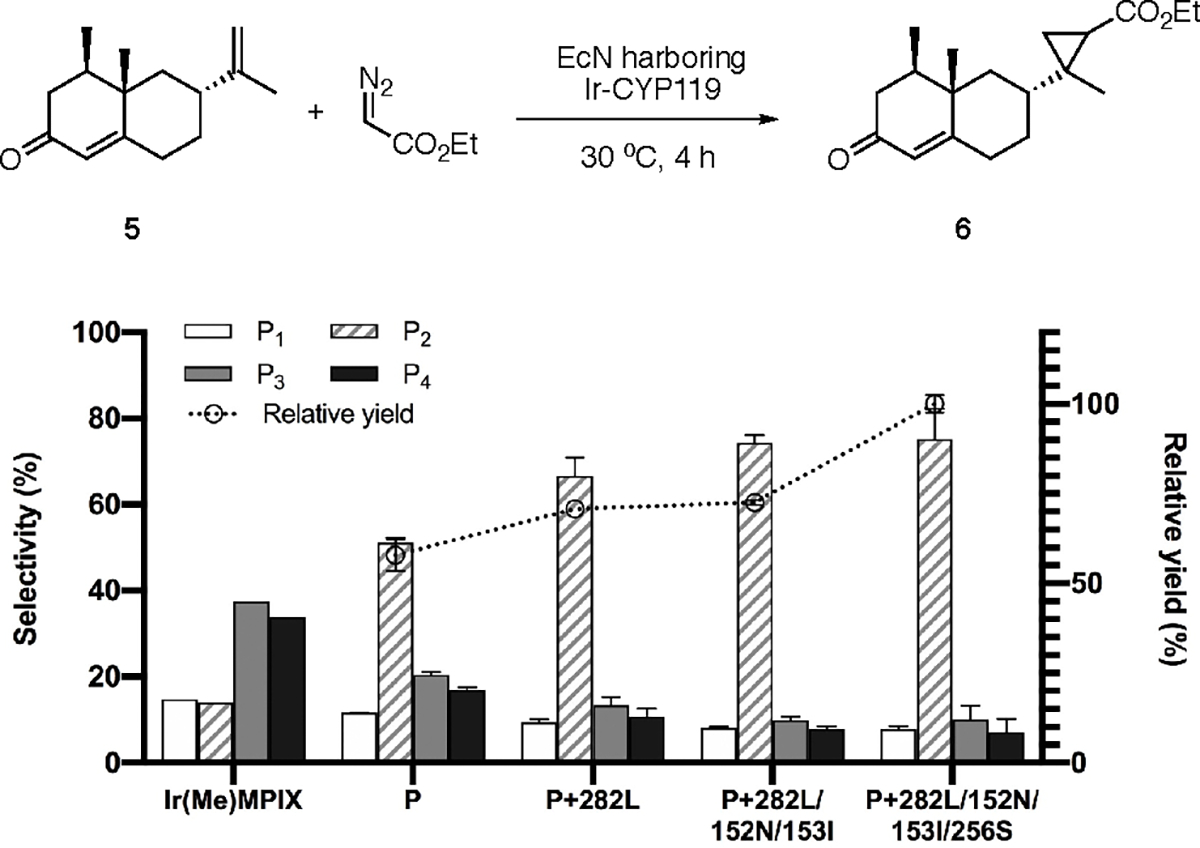
Directed evolution of Ir-CYP119 for diastereoselective cyclopropanation of (+)-nootkatone 5 with EcN as a screening platform. Reaction conditions: EcN expressing CYP119 mutant, OD600 ~30, 5 (2 μmol), EDA (16 μmol), DMSO (20 μL), M9-N (300 μL), 30 °C, 4 h. The cells were cultivated with 0.1 ppm of Ir(Me)MPIX during protein expression. P1–P4 are the four diastereomeric products numbered in the order of elution by gas chromatography. The selectivity of each diastereomer corresponds to its percent in the total four diastereomeric products. The relative yield is calculated by normalizing the yield of the reaction catalyzed by the final mutant as 100%. All data from the reactions in EcN are shown as the average from three biological replicates, with error bars indicating 1 standard deviation.
