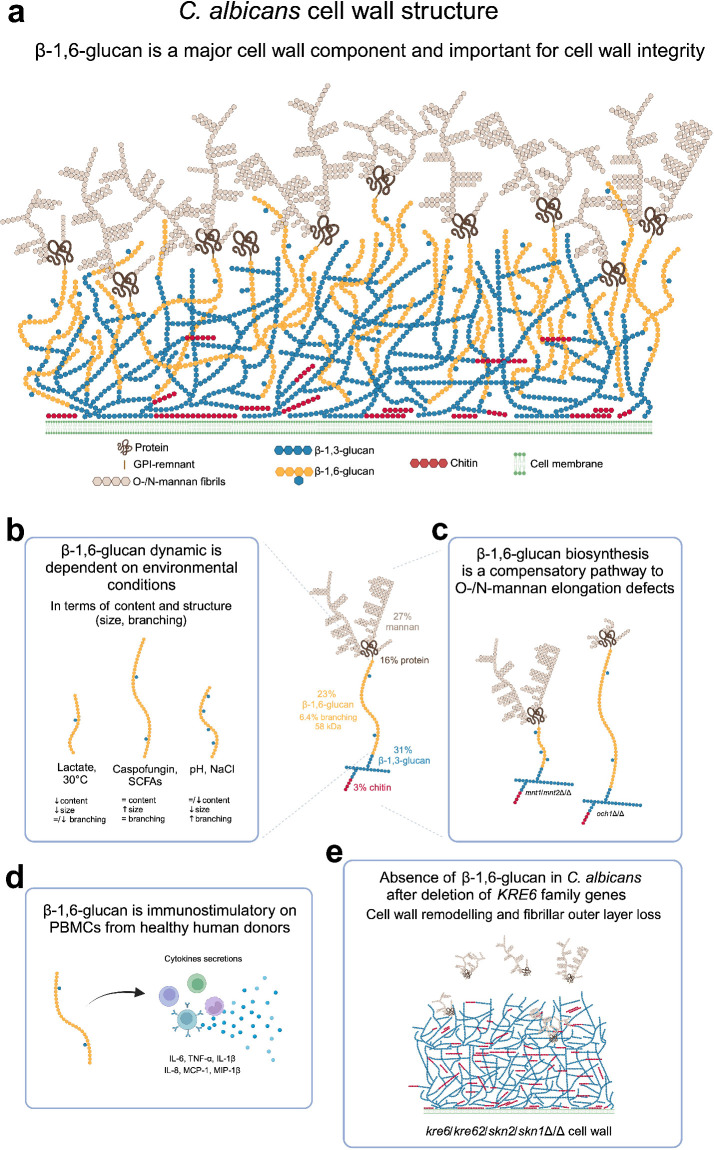
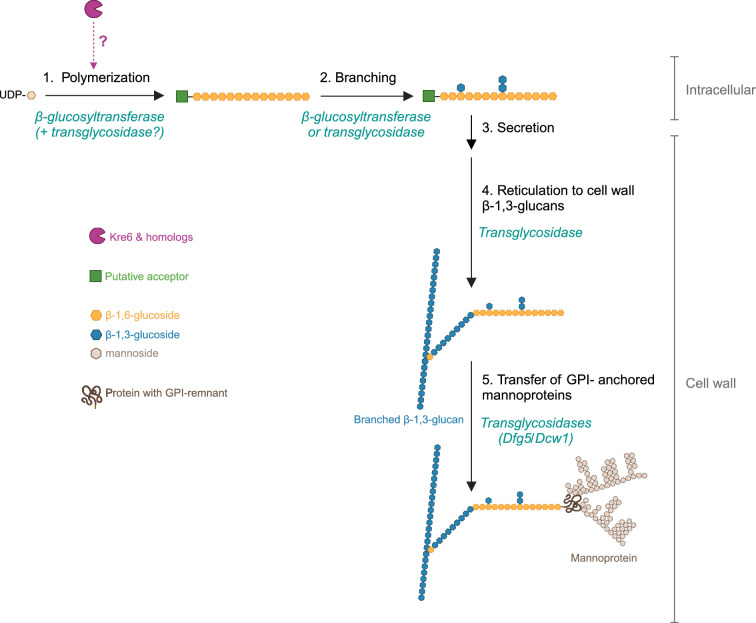
The cellular location of β-1,6-glucan synthesis in yeast is still unknown. We assume that synthesis begins intracellularly with the polymerization of linear β-1,6-glucan chain (step 1), which requires a β-glucosyltransferase and UDP-glucose as a donor (
Aimanianda et al., 2009;
Vink et al., 2004) and a putative acceptor (sugar, protein, lipid). Our data (
Figure 3g,
Figure 3—figure supplement 3) suggest that Kre6 and its homologs (Kre62, Skn1, Skn2) act at this stage, but their function remains unknown. Two proposed hypotheses are (1) Kre6 family members are β-glucosyltransferases and (2) they have glycosylhydrolase and transglycosidase activity essential for polymerization. Step 2 is the branching of nascent β-1,6-glucan where glucosides and laminaribiosides are added to form side chains. The enzymes (β-glucosyltransferase or transglycosidase) involved in this branching remain unknown. According to our data, members of the Kre6 family are not involved in branching (
Figure 3i). Next, the polysaccharide is secreted (step 3) and then cross-linked to β-1,3-glucans in the cell wall space by an unknown transglycosidase (step 4). The transfer of GPI-anchored proteins onto β-1,6-glucan (step 5), leading to the formation of the outer layer of the cell wall, appears to be driven by Dfg5/Dcw1 (
Vogt et al., 2020). The chronology between these two cross-links (steps 4 and 5) has not been established. Created with
BioRender.com.