Abstract
Purpose
Despite the emerging biologics, biomarkers and treatment options for asthma–chronic obstructive pulmonary disease (COPD) overlap (ACO) are still limited, requiring further research.
Methods
We enrolled 378 ACO patients from a multicenter real-world asthma cohort in Korea and compared the clinical characteristics, lung function, and exacerbation between type 2 (T2)-high and T2-low groups. We used the following comparisons: 1) low vs. high immunoglobulin E (IgE) group (≥ 100 IU/mL), 2) non-atopy vs. atopy group (sensitized to aeroallergen), 3) low vs. high blood eosinophil group (≥ 150/µL), and 4) low vs. high sputum eosinophil group (≥ 2%).
Results
The high sputum eosinophil ACO group (n = 37) showed significantly lower pre- and post-bronchodilator (BD) forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity (FVC) (45.7% ± 15.8% vs. 55.9% ± 16.2%, P = 0.016; 1.3 ± 0.6 L vs. 1.6 ± 0.5 L, P = 0.013 for pre-BD FEV1; 0.53 ± 0.1 vs. 0.59 ± 0.1, P = 0.018 for post-BD FEV1/FVC) than the low sputum eosinophil ACO group (n = 25). When examining changes in lung function at the 3-month follow-up, there were significant decreases in FEV1 in the high IgE ACO group (n = 104; −11.4% ± 16.7% vs. −4.4% ± 9.2%, P = 0.023) and ΔFEV1/FVC in the high sputum eosinophil ACO group (−0.049 ± 0.063 vs. −0.004 ± 0.064, P = 0.049) than in the low IgE ACO group (n = 44) and in the low sputum eosinophil ACO group, respectively. The risk of asthma exacerbation was significantly higher in the atopic ACO group (odds ratio, 4.2; 95% confidence interval, 1.0–17.4; P = 0.049) in the adjusted model.
Conclusions
Since ACOs with T2-high profiles may have lower lung function and more frequent exacerbations, T2-high specific therapies, such as biologics, should be actively considered in T2-high ACO patients.
Keywords: Asthma-COPD overlap, phenotype, biomarkers, inflammation, immunoglobulin E, atopy, eosinophils, cohort study, adult, Koreans
INTRODUCTION
Asthma and chronic obstructive pulmonary disease (COPD) are the major 2 chronic obstructive airway diseases, and the prevalence of asthma and COPD is reported to be 1% to 29%1 and 10.3%, respectively.2,3 Although there has been a perspective, such as the Dutch hypothesis, that they are located on a continuum and share some pathogenic mechanisms, asthma and COPD are currently considered distinct disease entities with separate approaches and treatments.4
Asthma is characterized by wheezing, dyspnea, chest tightness, and cough as the main symptoms and its diagnosis requires proven airway hyperresponsiveness or variable airway obstruction.1 This is caused by chronic airway inflammation, mainly due to a type 2 (T2) immune response, including eosinophils, mast cells, basophils, and type 2 innate lymphoid cells, and cytokines such as interleukin (IL)-4, IL-5, and IL-13.1
COPD is a progressive, fixed airway obstructive airway disease mainly caused by exposure to harmful biomass smoke and other environmental factors, and includes not only chronic bronchitis but also emphysematous changes.4 Cigarette smoking is the leading cause of COPD, but exposure to air pollution, secondhand smoke, and workplace dust and chemicals can also contribute to the disease.4,5 Although an increased level of macrophages and neutrophils in the sputum and bronchoalveolar lavage of COPD patients was reported, the pathogenesis of COPD is still unclear.5
Despite the differences between asthma and COPD, physicians have recognized a heterogeneous condition that overlaps both diseases, called asthma–COPD overlap (ACO), which affects 2% to 4% of the general population.6,7 For example, some asthmatics have fixed airway obstruction and significant smoking history, while some COPD patients have increased levels of typical asthma biomarkers such as eosinophils or sensitization to aeroallergens.4,7
Although precision medicine has opened a new era in asthma treatment with the application of biologics based on inflammatory biomarkers, treatment options for ACO are currently limited. Therefore, research on ACO using previously known T2 inflammatory biomarkers should be the starting point. In this study, we selected ACO patients from a multicenter real-world asthma cohort in Korea based on the presence of a smoking history and fixed airway obstruction. Then, we divided them into T2-high and T2-low groups using various T2 biomarkers and compared their clinical characteristics, lung function, symptom scores, and exacerbation rates.
MATERIALS AND METHODS
Study population
The COhort for Reality and Evolution of adult Asthma in Korea (COREA) is a representative multicenter asthma cohort in Korea that began in 2005.8 Adult asthmatic patients (≥ 18 years old) were recruited from 39 tertiary referral centers by specialists in allergy or pulmonology.8 Asthma was defined as the presence of at least one symptom, such as dyspnea, cough, or wheezing, along with evidence of airway hyperresponsiveness (AHR) or airway reversibility. AHR was confirmed by a bronchial provocation test using methacholine chloride when the provocative concentration causing a 20% fall in forced expiratory volume in 1 second (FEV1) (PC20) was less than 25 mg/mL, and airway reversibility was determined by an increase in FEV1 by at least 12% after inhalation of 400 μg of salbutamol or 4 weeks of anti-inflammatory treatments with inhaled or systemic steroids.
We obtained the results of AHR and airway reversibility by reviewing electronic medical records, and patients with asthma at any Global Initiative for Asthma (GINA) step could be enrolled. Physicians followed their usual clinical practice, treating asthma patients according to the GINA strategy at that time and adjusting medications based on symptoms, physical examination, and spirometry every three months.
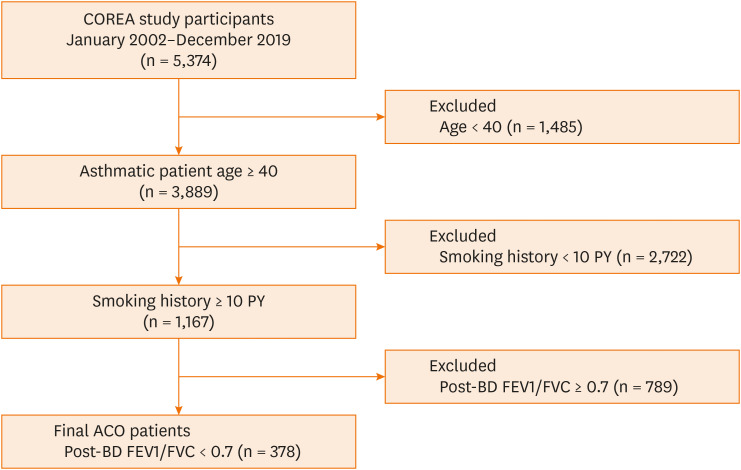
We selected ACO patients from the COREA registrants using the following criteria: 1) 40 years of age or older, 2) a smoking history of at least 10 pack-years, and 3) fixed airway obstruction of a post-bronchodilator (BD) FEV1/forced vital capacity (FVC) < 0.7.9,10 Finally, a total of 378 ACO patients were enrolled (Fig. 1).
Fig. 1. Study population flow chart.
COREA, COhort for Reality and Evolution of adult Asthma in Korea; PY, pack-year; ACO, asthma–chronic obstructive pulmonary disease overlap; BD, bronchodilator; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
This study was approved by the Institutional Review Boards of Ewha Womans University Medical Center (SEUMC 2020-08-022-009) and Asan Medical Center (2012-0234).
Study exposure
We divided the ACO patients into groups based on the following T2 inflammatory markers: 1) low immunoglobulin E (IgE) vs. high IgE group (≥ 100 IU/mL),11 2) non-atopy vs. atopy group (sensitized to aeroallergens by a skin prick test or multiple allergen simultaneous test), 3) low blood eosinophil vs. high blood eosinophil group (≥ 150/µL),1 and 4) low sputum eosinophil vs. high sputum eosinophil group (≥ 2%).1 Furthermore, we also investigated high blood eosinophil and high sputum eosinophil patients, high blood eosinophil and atopic patients, and high blood eosinophil and high IgE patients by combining these conditions. These inflammatory markers were assessed at enrollment, and we allowed the use of preexisting results by reviewing electronic medical records. T2 inflammatory markers can be measured in both stable and exacerbated states in routine practice. We selected positive results among multiple measurements, as these markers can vary over time. The GINA guidelines also recommend multiple checks of T2 inflammatory markers.1
Outcomes
We defined an asthma exacerbation as an unexpected outpatient clinic visit, emergency room visit, hospitalization, or the use of a steroid burst, which is the use of more than 30 mg/day of prednisolone or its equivalent for more than 3 days, during patients’ regular clinic visits every 3 months or at unscheduled visits due to exacerbation.12,13 We compared the differences (Δ) in FEV1 and FEV1/FVC between groups at baseline and after 3 months among subjects with available 3-month follow-up lung function results.
Statistical analyses
General characteristics among the study population were assessed using Student’s t-test or Mann Whitney U-test for continuous variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables. To estimate odds ratios (ORs) for asthma exacerbation according to T2 inflammatory status in ACO patients, we performed binary logistic regression (univariate and multivariate analyses) in the following ways: no adjustment, confounder adjustment for age and body mass index (BMI; model 1), and for age, BMI, and blood eosinophil count (model 2). The Kaplan–Meier method was used for time analysis of the first asthma exacerbation, and the strength of associations was measured as ORs or hazard ratios (HRs) and 95% confidence intervals (CIs). A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
General characteristics
A total of 378 ACO patients were enrolled, and their general characteristics are presented in Table 1. When comparing low IgE (n = 44) vs. high IgE patients (n = 104), allergic rhinitis (AR) was more common in the high IgE group (25.0% vs. 6.8%, P = 0.002) and blood eosinophil levels were also higher (4.7% ± 4.1% vs. 3.3% ± 2.4%, P = 0.016; 356.8 ± 320.8/µL vs. 242.9 ± 169.7/µL, P = 0.007). When comparing the non-atopic (n = 95) to the atopic patients (n = 79), AR was more common (31.7% vs. 11.6%, P = 0.002), and eosinophil counts were higher (436.0 ± 589.0/µL vs. 282.9 ± 259.2/µL, P = 0.041) in the atopic patients. The high blood eosinophil group (n = 210) had fewer women (6.2% vs. 13.6%, P = 0.048), more allergic rhinitis (25.2% vs. 14.6%, P = 0.045), higher serum total IgE (685.0 ± 1,151.4 IU/mL vs. 340.1 ± 526.1 IU/mL, P = 0.016), and lower total white blood cell (8,098.4 ± 2,056.0/µL vs. 8,845.2 ± 3,497.8/µL, P = 0.047) and neutrophil counts (4,623.4 ± 2,433.3/µL vs. 7,392.6 ± 12,086.3/µL, P = 0.028) than the low eosinophil patients (n = 103). The high sputum eosinophil group (n = 37) showed fewer AR (16.2% vs. 44.0%, P = 0.034), higher blood eosinophil levels (4.7% ± 5.0% vs. 2.7% ± 2.8%, P = 0.047; 390.6 ± 394.6/µL vs. 207.3 ± 181.6/µL, P = 0.018), and lower pre- and post-BD FEV1 and FEV1/FVC (45.7% ± 15.8% vs. 55.9% ± 16. 2%, P = 0.016; 1.3 ± 0.6 L vs. 1.6 ± 0.5 L, P = 0.013 for pre-BD FEV1; 53.1 ± 9.2 vs. 58.7 ± 8.3, P = 0.018 for post-BD FEV1/FVC) than the low sputum eosinophil group (n = 25). The more detailed characteristics of the total study population are described in Supplementary Table S1.
Table 1. Characteristics of study participants.
| Variable | Low IgE ACO (n = 44) | High IgE ACO (n = 104) | P value | Atopy (−) ACO (n = 95) | Atopy (+) ACO (n = 79) | P value | Low blood eosinophil ACO (n = 103) | High blood eosinophil ACO (n = 210) | P value | Low sputum eosinophil ACO (n = 25) | High sputum eosinophil ACO (n = 37) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | 63.0 ± 11.0 | 61.6 ± 9.7 | 0.463 | 63.4 ± 9.4 | 60.4 ± 9.9 | 0.046 | 64.6 ± 9.9 | 62.2 ± 10.3 | 0.047 | 61.0 ± 9.6 | 65.0 ± 11.9 | 0.168 |
| Male | 37 (84.1%) | 96 (92.3%) | 0.224 | 84 (88.4%) | 74 (93.7%) | 0.353 | 89 (86.4%) | 197 (93.8%) | 0.048 | 24 (96.0%) | 34 (91.9%) | 0.905 |
| BMI (kg/m2) | 24.7 ± 3.0 | 24.1 ± 2.7 | 0.285 | 24.4 ± 3.0 | 24.5 ± 2.5 | 0.719 | 24.0 ± 3.1 | 24.6 ± 3.9 | 0.143 | 24.2 ± 2.7 | 25.6 ± 7.4 | 0.317 |
| Smoking (pack-year) | 35.5 ± 24.49 | 34.6 ± 21.0 | 0.836 | 37.0 ± 21.7 | 31.4 ± 17.8 | 0.067 | 33.7 ± 20.2 | 36.0 ± 22.8 | 0.388 | 36.3 ± 15.5 | 35.3 ± 18.9 | 0.836 |
| Allergic rhinitis | 3 (6.8%) | 26 (25.0%) | 0.02 | 11 (11.6%) | 25 (31.7%) | 0.002 | 15 (14.6%) | 53 (25.2%) | 0.045 | 11 (44.0%) | 6 (16.2%) | 0.034 |
| WBC (/µL) | 7,707.1 ± 2,325.9 | 8,161.7 ± 2,558.2 | 0.314 | 8,224.3 ± 2,696.4 | 8,259.6 ± 2,471.9 | 0.932 | 8,845.2 ± 3,497.8 | 8,098.4 ± 2,056.0 | 0.047 | 8,973.0 ± 3,130.3 | 8,967.6 ± 3,056.6 | 0.995 |
| Eosinophil (/µL) | 242.9 ± 169.7 | 356.8 ± 320.8 | 0.007 | 282.9 ± 259.2 | 436.0 ± 589.0 | 0.041 | 81.1 ± 45.9 | 484.9 ± 467.5 | N/A | 207.3 ± 181.6 | 390.6 ± 394.6 | 0.018 |
| Neutrophil (/µL) | 4,452.5 ± 1,793.4 | 6,111.7 ± 12,225.6 | 0.202 | 4,900.7 ± 2,304.8 | 4,856.4 ± 2,093.8 | 0.902 | 7,392.6 ± 12,086.3 | 4,623.4 ± 2,433.3 | 0.028 | 6,059.5 ± 3,117.7 | 5,346.1 ± 2,555.6 | 0.347 |
| Serum total IgE (IU/mL) | 52.0 ± 30.4 | 788.4 ± 1,110.9 | 0 | 504.8 ± 799.7 | 521.0 ± 817.6 | 0.921 | 340.1 ± 526.1 | 685.0 ± 1,151.4 | 0.016 | 279.6 ± 252.0 | 449.7 ± 810.1 | 0.385 |
| Sputum eosinophil (%) | 10.1 ± 9.5 | 12.0 ± 15.5 | 0.719 | 13.9 ± 21.0 | 15.6 ± 17.0 | 0.791 | 8.0 ± 19.6 | 14.6 ± 20.3 | 0.252 | 0.3 ± 0.5 | 20.2 ± 22.5 | N/A |
| Sputum neurophil (%) | 36.0 ± 30.5 | 25.6 ± 25.4 | 0.314 | 42.6 ± 34.0 | 30.9 ± 28.9 | 0.263 | 43.5 ± 36.1 | 41.8 ± 32.9 | 0.862 | 49.5 ± 38.8 | 40.2 ± 31.0 | 0.302 |
| FeNO (ppb) | 26.0 ± 18.4 | 86.80 ± 60.2 | 0.24 | 41.3 ± 7.2 | 43.0 ± 31.7 | 0.879 | 50.0 ± 39.8 | 50.4 ± 43.7 | 0.986 | 38.3 ± 43.1 | 32.2 ± 11.26 | 0.728 |
| Pre-BD FEV1 (%) | 60.3 ± 16.7 | 54.5 ± 18.4 | 0.073 | 57.6 ± 18.9 | 54.2 ± 17.0 | 0.218 | 58.0 ± 18.8 | 55.6 ± 17.7 | 0.27 | 56.0 ± 16.2 | 45.7 ± 15.8 | 0.016 |
| Pre-BD FEV1 (L) | 1.7 ± 0.6 | 1.7 ± 0.7 | 0.473 | 1.7 ± 0.6 | 1.7 ± 0.7 | 0.846 | 1.7 ± 0.6 | 1.7 ± 0.6 | 0.961 | 1.6 ± 0.5 | 1.3 ± 0.6 | 0.013 |
| Post-BD FEV1/FVC | 55.0 ± 8.6 | 53.9 ± 8.9 | 0.495 | 54.8 ± 9.1 | 54.9 ± 8.9 | 0.905 | 55.1 ± 9.5 | 54.4 ± 9.1 | 0.557 | 58.7 ± 8.3 | 53.1 ± 9.2 | 0.018 |
| ACT scores (total 25) | 21.5 ± 3.6 | 21.1 ± 3.7 | 0.711 | 20.3 ± 4.6 | 21.3 ± 3.0 | 0.318 | 19.7 ± 5.3 | 20.0 ± 4.2 | 0.761 | 16.3 ± 6.1 | 15.8 ± 5.9 | 0.835 |
Data are presented as number (%) or mean ± standard deviation. Bold-styled values denote significant ones.
IgE, immunoglobulin E; ACO, asthma-chronic obstructive pulmonary disease overlap; BMI, body mass index; WBC, white blood cell; N/A, not applicable; FeNO, fractional exhaled nitric oxide; BD, bronchodilator; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ACT, asthma control test.
Characteristics according to the combination of each T2 biomarker
The high blood and sputum eosinophil patients (n = 30) had lower neutrophil counts compared to the low group (n = 11; 4,496.7 [interquartile range {IQR} 3,444.0, 6,060.5] vs. 6,822.0/µL, [IQR 4,765.6, 8,382.0], P = 0.11), and had lower post-BD FEV1 (49.6% ± 17.3% vs. 62.3% ± 16.4%, P = 0.04) and post-BD FEV1/FVC (52.8 ± 8.9 vs. 59.7 ± 6.8, P = 0.025). The high blood eosinophil and atopic group (n = 56) was younger (58.2 ± 10.0 years vs. 63.8 ± 10.9 years, P = 0.019), male-predominant (54 [96.4%] vs. 24 [80.0%], P = 0.035), and more likely to have allergic rhinitis (35.7% vs. 10.0%, P = 0.021), while it also had lower neutrophils (4,693.3 ± 1,576.7/µL vs. 5,904.6 ± 2,962.7/µL, P = 0.047), lower pre-BD FEV1 (54.1 ± 16.9% vs. 63.0 ± 22.3%, P = 0.043), and fewer hospitalizations for exacerbations per year (0.3 ± 0.6 vs. 0.9 ± 0.7, P = 0.036) than the low group (n = 30). The high blood eosinophil and high IgE patients (n = 70) had lower neutrophils (55.6% ± 10.1% vs. 65.4% ± 11.1%, P = 0.001) and lower pre-BD FEV1 (52.4% ± 17.8% vs. 62.4% ± 18.3%, P = 0.041) than the low group (n = 17; Supplementary Table S2). There were no significant differences in asthma medication use among the groups (Supplementary Table S3). Among the 378 participants, there was 1 omalizumab user and 1 reslizumab user.
Changes in lung function and ACT score at the 3-month follow-up
When examining changes in the lung function and asthma control test (ACT) score at the 3-month follow-up, there were significant decreases in FEV1 in the high IgE group (−11.4% ± 16.7% vs. −4.4% ± 9.2%, P = 0.023) and ΔFEV1/FVC in the high sputum eosinophil group (−4.9 ± 6.3 vs. −0.4 ± 6.4, P = 0.049) than in the low IgE and low sputum eosinophil groups, respectively (Table 2). Otherwise, there were no significant differences.
Table 2. The changes in lung function and ACT score at the 3-month follow-up.
| Variable | Low IgE ACO (n = 22) | High IgE ACO (n = 60) | P value | Atopy (−) ACO (n = 60) | Atopy (+) ACO (n = 47) | P value | Low blood eosinophil ACO (n = 62) | High blood eosinophil ACO (n = 116) | P value | Low sputum eosinophil ACO (n = 25) | High sputum eosinophil ACO (n = 37) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔFEV1 (mL) | −0.2 ± 0.5 | −0.3 ± 0.5 | 0.467 | −0.4 ± 0.6 | −0.5 ± 0.5 | 0.589 | −0.3 ± 0.6 | −0.3 ± 0.5 | 0.583 | −0.3 ± 0.3 | −0.3 ± 0.3 | 0.995 |
| ΔFEV1 (%) | −4.4 ± 9.2 | −11.4 ± 16.7 | 0.023 | −11.9 ± 18.0 | −13.5 ± 15.8 | 0.628 | −11.0 ± 17.3 | −9.5 ± 14.9 | 0.549 | −8.4 ± 13.1 | −11.4 ± 11.2 | 0.488 |
| ΔFEV1/FVC | −4.8 ± 12.0 | −6.1 ± 12.9 | 0.682 | −7.2 ± 9.6 | −6.1 ± 10.5 | 0.578 | −5.5 ± 11.5 | −5.3 ± 10.3 | 0.885 | −0.4 ± 6.4 | −4.9 ± 6.3 | 0.049 |
| ΔACT | −0.8 ± 1.0 | −0.2 ± 5.3 | 0.685 | −1.3 ± 5.3 | −0.6 ± 4.5 | 0.757 | −1.8 ± 6.3 | −1.1 ± 3.6 | 0.654 | −3.3 ± 4.5 | −5.0 ± 10.4 | 0.809 |
The changes (Δ) were calculated by 3 months to baseline. P value was calculated with Student’s t-test for continuous variables. Bold-styled values denote significant ones.
ACT, asthma control test; IgE, immunoglobulin E; ACO, asthma–chronic obstructive pulmonary disease overlap; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
The risk of asthma exacerbations
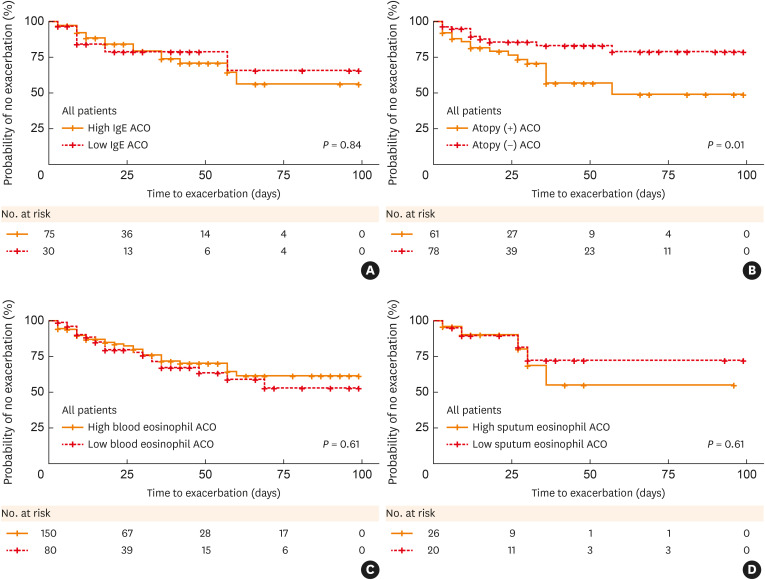
We investigated the risk of annual asthma exacerbations among the groups. The proportion of individuals experiencing exacerbations was significantly higher in the atopic group compared to the non-atopic group (60.8% vs. 29.4%, P = 0.025), with no significant differences in the other groups. The risk of asthma exacerbation was also significantly higher in atopic patients in the unadjusted model (OR, 3.7; 95% CI, 1.1–12.2; P = 0.03), and this remained significant after adjustment (OR, 4.2; CI, 1.0–17.4; P = 0.049; Table 3). Next, we examined the time to first asthma exacerbation (TFE) in each group using the Kaplan–Meier method. The atopic group had a shorter TFE than the non-atopic group (P = 0.01; Fig. 2), yielding an HR 0.39 for TEF (95% CI, 0.18–0.82; P = 0.013). There was no significant difference in TFE among the other groups (Supplementary Fig. S1).
Table 3. Risk of asthma exacerbation in T2-high ACO and T2-low ACO.
| Acute exacerbation | Model 1: unadjusted | Model 2: age, BMI adjusted | Model 3: age, BMI, blood eosinophil adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P value | Adjusted OR | 95% CI | P value | Adjusted OR | 95% CI | P value | |
| Low IgE ACO | Reference | Reference | Reference | ||||||
| High IgE ACO | 0.47 | 0.05–4.43 | 0.507 | 0.52 | 0.05–5.33 | 0.579 | 0.54 | 0.05–5.78 | 0.503 |
| Atopy (−) ACO | Reference | Reference | Reference | ||||||
| Atopy (+) ACO | 3.72 | 1.14–12.17 | 0.03 | 3.87 | 1.09–13.68 | 0.036 | 4.19 | 1.01–17.42 | 0.049 |
| Low blood eosinophil ACO | Reference | Reference | Reference | ||||||
| High blood eosinophil ACO | 0.84 | 0.33–2.12 | 0.715 | 0.8 | 0.3–2.12 | 0.653 | 1.47 | 0.19–11.35 | 0.713 |
All adjusting variables were measured at the baseline visit. Bold-styled values denote significant ones.
T2, type 2; ACO, asthma–chronic obstructive pulmonary disease overlap; BMI, body mass index; OR, odds ratio; CI, confidence interval; IgE, immunoglobulin E.
Fig. 2. Kaplan–Meier curves for the time to the first asthma exacerbation, according to each group.
(A) High IgE ACO vs. Low IgE ACO. (B) Atopic ACO vs. Non-atopic ACO. (C) High blood eosinophilic ACO vs. Low blood eosinophilic ACO. (D) High sputum eosinophilic ACO vs. Low sputum eosinophilic ACO.
IgE, immunoglobulin E; ACO, asthma–chronic obstructive pulmonary disease overlap.
DISCUSSION
In this study of ACO patients, high sputum eosinophil patients showed decreased lung function, with lower FEV1 and FEV1/FVC. When combining each T2 marker, the high blood and sputum eosinophil, high blood eosinophil and atopic, and high blood eosinophil and high IgE patients showed a lower FEV1. We found that patients with T2 dominance demonstrated a more pronounced decrease in lung function. At the 3-month follow-up, high IgE patients showed a significant decrease in FEV1, and high sputum eosinophil patients exhibited a significantly greater decrease in FEV1/FVC. Here, as well, some T2-dominant patients showed a more pronounced decline in lung function. Regarding exacerbations, atopic patients had a higher risk of asthma exacerbation and a shorter time to first exacerbation. Collectively, among all of the groups, patients with T2 dominance showed lower lung function, a more pronounced decline in lung function, and a higher risk of exacerbation.
ACO has various definitions, and from an asthma perspective, it is generally defined as a persistent airflow limitation with a post-BD FEV1/FVC < 0.7 in individuals aged 40 or older, having a history of smoking at least 10 pack-years or more, or significant other biomass smoke exposure.9,14,15 In the COPD population, ACO can usually be characterized by a substantial bronchodilator response (an increase in FEV1 of over 400 mL and 15%), the presence of atopy, elevated serum IgE levels, sputum eosinophils of 2% or more, or an increase in fractional exhaled nitric oxide (FeNO) levels. It denotes COPD accompanied by typical asthma features.9,14,15
ACO patients are known to exhibit more wheezing and sputum production compared to patients with asthma or COPD alone and have been reported to have more severe dyspnea as measured by the Medical Research Council score.16,17,18 Meta-analyses indicate a higher exacerbation rate in ACO than in asthma alone or COPD.16,19,20 Patients with eosinophilic ACO have been reported to have significantly higher rates of asthma exacerbations and oral corticosteroid (OCS) requirements compared to patients with severe eosinophilic asthma only.21 Regarding lung function, there are studies reporting no significant differences in FEV1 decline among ACO, asthma, and COPD,17,18 while another study suggested a slower decline in ACO.22 Additionally, the Copenhagen City Heart Study revealed a lower FEV1 decline in ACO with concomitant early-onset asthma and a higher FEV1 decline in ACO with late-onset asthma.23
The absence of a universally accepted definition for ACO poses a challenge in comparing research and study outcomes. Thus far, comparative studies have mainly compared ACO to asthma or COPD, and there is a lack of comparison within ACO based on phenotypes or endotypes. In this regard, this study aimed to investigate whether there are differences in clinical manifestations among ACO phenotypes and endotypes using established T2 biomarkers, revealing that overall, T2-dominant ACO tends to be more severe in terms of lung function, lung function decline, and exacerbations. In particular, individuals with ACO and high sputum eosinophil levels demonstrated lower lung function, with a faster decline in FEV1/FVC. ACO patients with high IgE levels exhibited a faster decline in FEV1, and in cases of atopic ACO, there was a higher risk of asthma exacerbation.
Blood and sputum eosinophil, one of the typical markers for T2-high asthma, are increasingly recognized for their importance in COPD.24,25 Elevated blood eosinophils have been significantly associated with acute COPD exacerbations, mortality, reduced FEV1, and responsiveness to inhaled corticosteroids (ICS) and systemic corticosteroids.26,27 FeNO is also a well-known biomarker for airway T2 inflammation.1 Previous studies indicated higher FeNO levels in COPD patients with concomitant allergic rhinitis,28 and COPD patients with consistently elevated FeNO levels above 20 ppb were at a higher risk of acute exacerbations.29 Additionally, severe COPD patients responding to ICS treatment exhibited higher FeNO levels.30 A meta-analysis showed that ACO patients, although lacking a standardized definition, demonstrated higher FeNO levels compared to those with COPD alone.31 However, a defined cut-off value to distinguish ACO from COPD has not been established.
Serum IgE serves as a marker associated with various typical allergic diseases such as allergic rhinitis, asthma, atopic dermatitis, and chronic urticaria.32 Studies focused on IgE in COPD populations are scarce. In a previous cross-sectional observational study, COPD accompanied by late-onset asthma exhibited elevated total IgE, whereas ACO with early-onset asthma showed relatively lower IgE levels.33 For allergic sensitization, so-called atopic, there is limited research on allergic sensitization in ACO, although one study reported more wheezing, night cough, and acute exacerbations in atopic COPD patients,34 when comparing COPD and atopic ACOs, there were lower FEV1 and more exacerbations in atopic ACOs.11
Recently, there has been an emergence of biologics in the treatment of severe asthma, in addition to the conventional use of medium-to-high dose ICS-long-acting β2 agonist.1,35 These biologics are now being considered based on various biomarkers, usually T2, to determine their administration and predict their treatment response.1,35 However, research on the use of biologics in ACO is currently limited.36 This is mainly due to the exclusion of ACO, rather than pure asthma or COPD, from most clinical trials on biologics.37
A previous study on omalizumab, an anti-IgE monoclonal antibody, showed comparable improvements in symptom scores and exacerbation rates up to 48 weeks in both asthma and ACO groups,38 while another study demonstrated that omalizumab did not show significant improvements in lung function in ACO compared to asthma.39 Mepolizumab, an anti-IL-5 monoclonal antibody, has been reported to reduce exacerbation rates and decrease OCS use in patients concurrently diagnosed with both severe asthma and COPD in Medicare data research.40 Other studies on mepolizumab reported positive responses in blood eosinophil levels, OCS use, and exacerbation rates in both asthma and ACO patients,41 and significant improvements in exacerbation rates and symptom scores in ACO patients.42 In COPD with increased eosinophils, mepolizumab demonstrated efficacy in reducing exacerbations compared to a placebo.43 Benralizumab, an anti-IL-5 receptor α monoclonal antibody, showed less clear effects on exacerbation reduction in moderate to severe COPD with elevated eosinophils,44 while other study reported the most significant benefit in patients with eosinophil counts above 220 cells/µL and severe airflow limitation.45 A phase 2 trial targeting patients with both asthma and COPD is currently being conducted (ClinicalTrials.gov Identifier: NCT04098718). For dupilumab, an anti-IL-4 receptor α monoclonal antibody blocking both IL-4 and IL-13, a study on moderate to severe COPD patients with elevated eosinophils showed better treatment responses in terms of exacerbation reduction, lung function improvement, and symptom scores compared to a placebo.46
This study has several limitations. First, ACO was defined based on smoking history and post-BD airway obstruction in the asthma cohort. The research from a COPD perspective on defining ACO may be necessary. Secondly, this study did not follow up on T2 inflammatory biomarkers but mostly measured them as one-time assessments. Further research on biomarkers for ACO is necessary. Additionally, this study was conducted exclusively on the Korean population.
The use of biologics in uncontrolled ACO is reported to be about three times lower than in asthma.47 Our study revealed that ACO patients with elevated T2 biomarkers show worse outcomes in baseline lung function, lung function decline, and exacerbations. Therefore, active consideration of add-on therapy, including biologics, is suggested for ACO patients.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C0481, HC20C0076).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Characteristics of total study participants
Characteristics of study participants according to combinations of T2 markers
Medication use in each ACO group
Kaplan–Meier curves for the time to the first asthma exacerbation, according to each group.
References
- 1.Global Initiative for Asthma. [place unknown]: Global Initiative for Asthma; 2024. Global strategy for asthma management and prevention. [Google Scholar]
- 2.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5:020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekov E, Nuñez A, Sin DD, Ichinose M, Rhee CK, Maselli DJ, et al. Update on asthma-COPD overlap (ACO): a narrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1783–1799. doi: 10.2147/COPD.S312560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399:2227–2242. doi: 10.1016/S0140-6736(22)00470-6. [DOI] [PubMed] [Google Scholar]
- 6.Morgan BW, Grigsby MR, Siddharthan T, Chowdhury M, Rubinstein A, Gutierrez L, et al. Epidemiology and risk factors of asthma-chronic obstructive pulmonary disease overlap in low- and middle-income countries. J Allergy Clin Immunol. 2019;143:1598–1606. doi: 10.1016/j.jaci.2018.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Khurana S, Federman AD, Wisnivesky J, Holguin F. Asthma-chronic obstructive pulmonary disease overlap. Immunol Allergy Clin North Am. 2020;40:565–573. doi: 10.1016/j.iac.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim TB, Park CS, Bae YJ, Cho YS, Moon HB COREA Study Group. Factors associated with severity and exacerbation of asthma: a baseline analysis of the COhort for Reality and Evolution of adult Asthma in Korea (COREA) Ann Allergy Asthma Immunol. 2009;103:311–317. doi: 10.1016/S1081-1206(10)60530-3. [DOI] [PubMed] [Google Scholar]
- 9.Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48:664–673. doi: 10.1183/13993003.00436-2016. [DOI] [PubMed] [Google Scholar]
- 10.Bonten TN, Kasteleyn MJ, de Mutsert R, Hiemstra PS, Rosendaal FR, Chavannes NH, et al. Defining asthma-COPD overlap syndrome: a population-based study. Eur Respir J. 2017;49:1602008. doi: 10.1183/13993003.02008-2016. [DOI] [PubMed] [Google Scholar]
- 11.Hersh CP, Zacharia S, Prakash Arivu Chelvan R, Hayden LP, Mirtar A, Zarei S, et al. Immunoglobulin E as a biomarker for the overlap of atopic asthma and chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis (Miami) 2020;7:1–12. doi: 10.15326/jcopdf.7.1.2019.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129:S34–S48. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calverley PM. Effect of corticosteroids on exacerbations of asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:161–166. doi: 10.1513/pats.200402-008MS. [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa S, Ichinose M. Definition and diagnosis of asthma-COPD overlap (ACO) Allergol Int. 2018;67:172–178. doi: 10.1016/j.alit.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Milne S, Mannino D, Sin DD. Asthma-COPD overlap and chronic airflow obstruction: definitions, management, and unanswered questions. J Allergy Clin Immunol Pract. 2020;8:483–495. doi: 10.1016/j.jaip.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Miravitlles M, Soriano JB, Ancochea J, Muñoz L, Duran-Tauleria E, Sánchez G, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107:1053–1060. doi: 10.1016/j.rmed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Brzostek D, Kokot M. Asthma-chronic obstructive pulmonary disease overlap syndrome in Poland. Findings of an epidemiological study. Postepy Dermatol Alergol. 2014;31:372–379. doi: 10.5114/pdia.2014.47120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pleasants RA, Ohar JA, Croft JB, Liu Y, Kraft M, Mannino DM, et al. Chronic obstructive pulmonary disease and asthma-patient characteristics and health impairment. COPD. 2014;11:256–266. doi: 10.3109/15412555.2013.840571. [DOI] [PubMed] [Google Scholar]
- 19.Menezes AMB, Montes de Oca M, Pérez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145:297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen M, Bårnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome--a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1443–1454. doi: 10.2147/COPD.S85363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiles SA, Gibson PG, McDonald VM. Disease burden of eosinophilic airway disease: comparing severe asthma, COPD and asthma-COPD overlap. Respirology. 2021;26:52–61. doi: 10.1111/resp.13841. [DOI] [PubMed] [Google Scholar]
- 22.Park HY, Lee SY, Kang D, Cho J, Lee H, Lim SY, et al. Favorable longitudinal change of lung function in patients with asthma-COPD overlap from a COPD cohort. Respir Res. 2018;19:36. doi: 10.1186/s12931-018-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange P, Çolak Y, Ingebrigtsen TS, Vestbo J, Marott JL. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med. 2016;4:454–462. doi: 10.1016/S2213-2600(16)00098-9. [DOI] [PubMed] [Google Scholar]
- 24.Bafadhel M, Pavord ID, Russell RE. Eosinophils in COPD: just another biomarker? Lancet Respir Med. 2017;5:747–759. doi: 10.1016/S2213-2600(17)30217-5. [DOI] [PubMed] [Google Scholar]
- 25.Terl M, Sedlák V, Cap P, Dvořáková R, Kašák V, Kočí T, et al. Asthma management: a new phenotype-based approach using presence of eosinophilia and allergy. Allergy. 2017;72:1279–1287. doi: 10.1111/all.13165. [DOI] [PubMed] [Google Scholar]
- 26.Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 28.Tamada T, Sugiura H, Takahashi T, Matsunaga K, Kimura K, Katsumata U, et al. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis. 2015;10:2169–2176. doi: 10.2147/COPD.S88274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcázar-Navarrete B, Ruiz Rodríguez O, Conde Baena P, Romero Palacios PJ, Agusti A. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur Respir J. 2018;51:1701457. doi: 10.1183/13993003.01457-2017. [DOI] [PubMed] [Google Scholar]
- 30.Kunisaki KM, Rice KL, Janoff EN, Rector TS, Niewoehner DE. Exhaled nitric oxide, systemic inflammation, and the spirometric response to inhaled fluticasone propionate in severe chronic obstructive pulmonary disease: a prospective study. Ther Adv Respir Dis. 2008;2:55–64. doi: 10.1177/1753465808088902. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Zhang M, Wang Y, Su X, Lei T, Yu H, et al. Diagnostic value of fractional exhaled nitric oxide in differentiating the asthma-COPD overlap from COPD: a systematic review and meta-analysis. Expert Rev Respir Med. 2022;16:679–687. doi: 10.1080/17476348.2022.2011221. [DOI] [PubMed] [Google Scholar]
- 32.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fingleton J, Huang K, Weatherall M, Guo Y, Ivanov S, Bruijnzeel P, et al. Phenotypes of symptomatic airways disease in China and New Zealand. Eur Respir J. 2017;50:1700957. doi: 10.1183/13993003.00957-2017. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson DB, Matsui EC, Belli A, McCormack MC, Peng E, Pierre-Louis S, et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:187–192. doi: 10.1164/rccm.201211-2103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019;144:1–12. doi: 10.1016/j.jaci.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Shim JS, Kim H, Kwon JW, Park SY, Kim S, Kim BK, et al. A comparison of treatment response to biologics in asthma-COPD overlap and pure asthma: findings from the PRISM study. World Allergy Organ J. 2023;16:100848. doi: 10.1016/j.waojou.2023.100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung C, Sin DD. Asthma-COPD overlap: what are the important questions? Chest. 2022;161:330–344. doi: 10.1016/j.chest.2021.09.036. [DOI] [PubMed] [Google Scholar]
- 38.Hanania NA, Chipps BE, Griffin NM, Yoo B, Iqbal A, Casale TB. Omalizumab effectiveness in asthma-COPD overlap: post hoc analysis of PROSPERO. J Allergy Clin Immunol. 2019;143:1629–1633.e2. doi: 10.1016/j.jaci.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 39.Maltby S, Gibson PG, Powell H, McDonald VM. Omalizumab treatment response in a population with severe allergic asthma and overlapping COPD. Chest. 2017;151:78–89. doi: 10.1016/j.chest.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 40.Casale T, Molfino NA, Silver J, Bogart M, Packnett E, McMorrow D, et al. Real-world effectiveness of mepolizumab in patients with severe asthma and associated comorbidities. Ann Allergy Asthma Immunol. 2021;127:354–362.e2. doi: 10.1016/j.anai.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Isoyama S, Ishikawa N, Hamai K, Matsumura M, Kobayashi H, Nomura A, et al. Efficacy of mepolizumab in elderly patients with severe asthma and overlapping COPD in real-world settings: a retrospective observational study. Respir Investig. 2021;59:478–486. doi: 10.1016/j.resinv.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Kritikos V, Harvey ES, Stevens S, Katelaris CH, Langton D, Rimmer J, et al. Comorbidities modify the phenotype but not the treatment effectiveness to mepolizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2023;11:885–895.e13. doi: 10.1016/j.jaip.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Pavord ID, Chanez P, Criner GJ, Kerstjens HA, Korn S, Lugogo N, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377:1613–1629. doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 44.Criner GJ, Celli BR, Brightling CE, Agusti A, Papi A, Singh D, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med. 2019;381:1023–1034. doi: 10.1056/NEJMoa1905248. [DOI] [PubMed] [Google Scholar]
- 45.Criner GJ, Celli BR, Singh D, Agusti A, Papi A, Jison M, et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: analyses of GALATHEA and TERRANOVA studies. Lancet Respir Med. 2020;8:158–170. doi: 10.1016/S2213-2600(19)30338-8. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt SP, Rabe KF, Hanania NA, Vogelmeier CF, Cole J, Bafadhel M, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med. 2023;389:205–214. doi: 10.1056/NEJMoa2303951. [DOI] [PubMed] [Google Scholar]
- 47.Reddel HK, Vestbo J, Agustí A, Anderson GP, Bansal AT, Beasley R, et al. Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur Respir J. 2021;58:2003927. doi: 10.1183/13993003.03927-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of total study participants
Characteristics of study participants according to combinations of T2 markers
Medication use in each ACO group
Kaplan–Meier curves for the time to the first asthma exacerbation, according to each group.