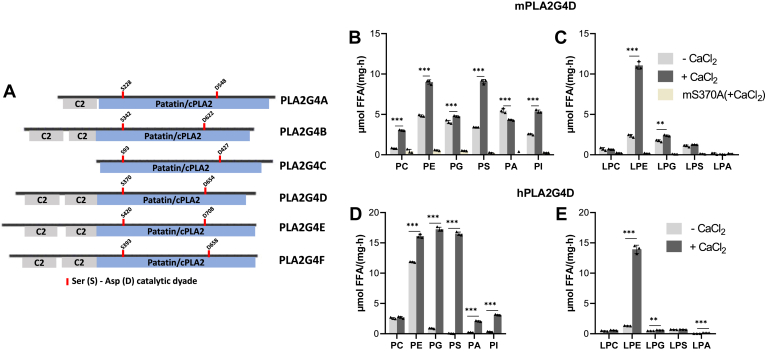
Fig. 1.
PLA2G4D acts as phospholipase with broad head group specificity. (A) Schematic illustration of the domain architecture of the mPLA2G4 family showing C2 domains (C2), the patatin-like domain (patatin/cPLA2), and the position of the serine (S) – aspartate (D) catalytic dyad. (B, C) (Lyso)phospholipase activity of partially purified murine PLA2G4D and the mS370A mutant in the absence and presence of 1 mM CaCl2. FFA release was determined using commercially available colorimetric kits. (D, E) (Lyso)phospholipase activity of partially purified human PLA2G4D in the absence and presence of 1 mM CaCl2. All enzyme activity assays were carried out by incubating 1 μg of partially purified protein with 20 μl of lipid substrate (1 mM) in PBS (pH 7.4) containing 2% BSA (FA free) for 1 h at 37°C (n = 3). Data are presented as mean ± SD. Statistical comparison was performed with multiple unpaired two-tailed Student’s t test, followed by Bonferroni posthoc analysis. Statistically significant differences are shown as: ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. PLA2G4, phospholipase A2 group IV.