Fig. 4.
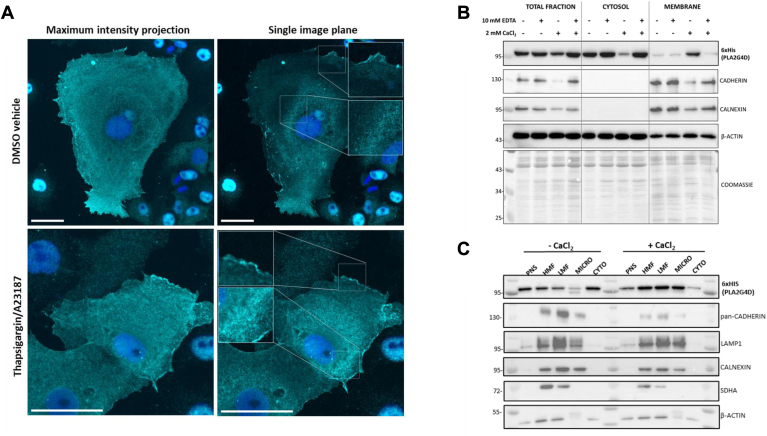
PLA2G4D is a cytosolic enzyme that interacts with membranes in a calcium-dependent manner. (A) Confocal imaging of COS7 cells stably overexpressing His-tagged mPLA2G4D. Cells were fixed with paraformaldehyde and stained with an anti-6xHis antibody (cyan). To increase cytosolic Ca2+ concentrations, cells were treated with thapsigargin/calcimycin A23187 (5 μM each) or DMSO for 15 min before fixation. Maximum intensity projection shows the highest pixel intensities from stacked images indicating that the enzyme shows primarily cytosolic localization. Zoomed sections from a single image plane suggest that PLA2G4D partially colocalizes with cytoplasmic and plasma membranes. Nuclei were stained with DAPI (blue). Scale bar: 40 μm. Data are representative for two independent experiments. (B) Western blotting analysis of total (1,000 g supernatant), cytosolic (100,000 g supernatant), and membrane fractions (100,000 g pellet) of COS7 cells overexpressing His-tagged mPLA2G4D. Cell fractionation was performed in the absence and presence of 2 mM CaCl2 and 10 mM EDTA. The plasma membrane protein Cadherin and ER protein calnexin were used as markers for the membrane fractions. β-Actin and Coomassie staining served as loading control. Data are representative for two independent experiments. (C) Western blotting analysis of total fraction (TF; 1,000 g supernatant), heavy mitochondrial fraction (HMF; 3,000 g pellet), light mitochondrial fraction (LMF; 17,000 g pellet), microsomal fraction (MICRO; 100,000 g pellet), and cytosolic fraction (CYTO; 100,000 g supernatant) of COS7 cells overexpressing His-tagged mPLA2G4D. Cell fractionation was performed in the absence and presence of 2 mM CaCl2. Lamp1, Calnexin, pan-Cadherin, and SDHA were used as marker-proteins for the endo-lysosomal compartment, ER, plasma membrane, and mitochondria, respectively. β-Actin served as loading control. PLA2G4, phospholipase A2 group IV.