Abstract
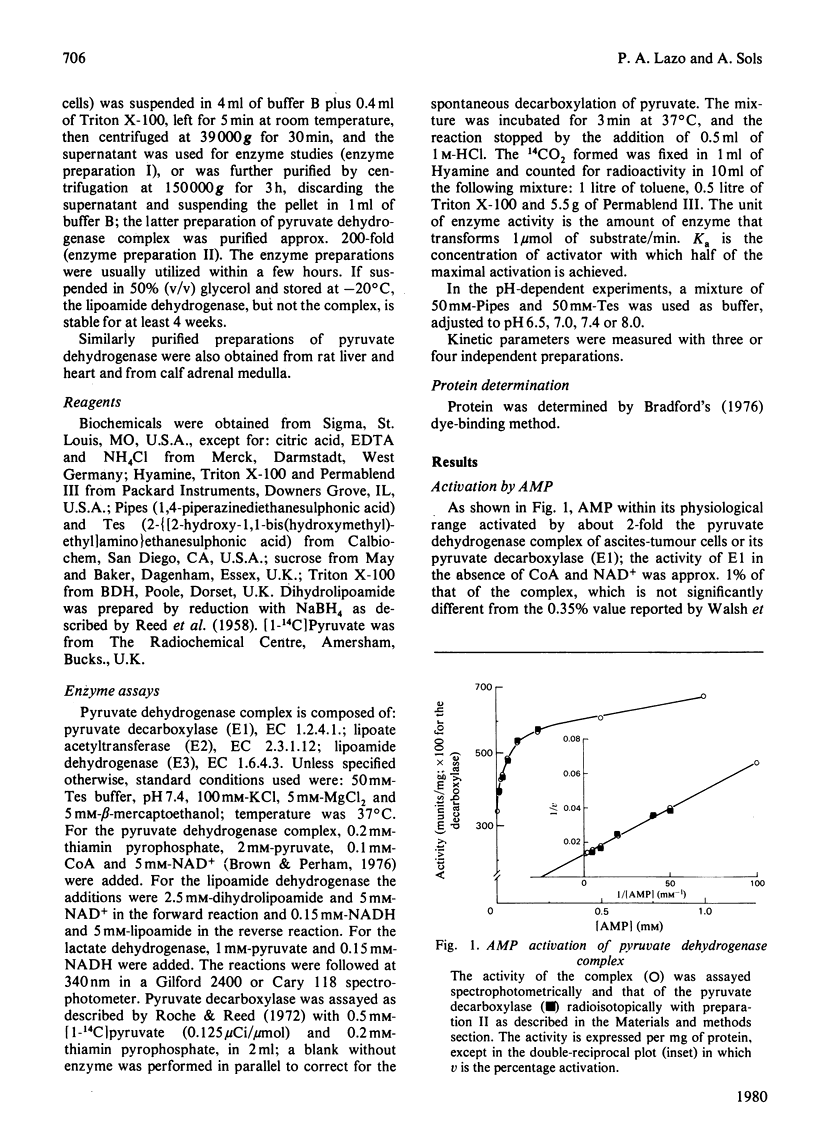
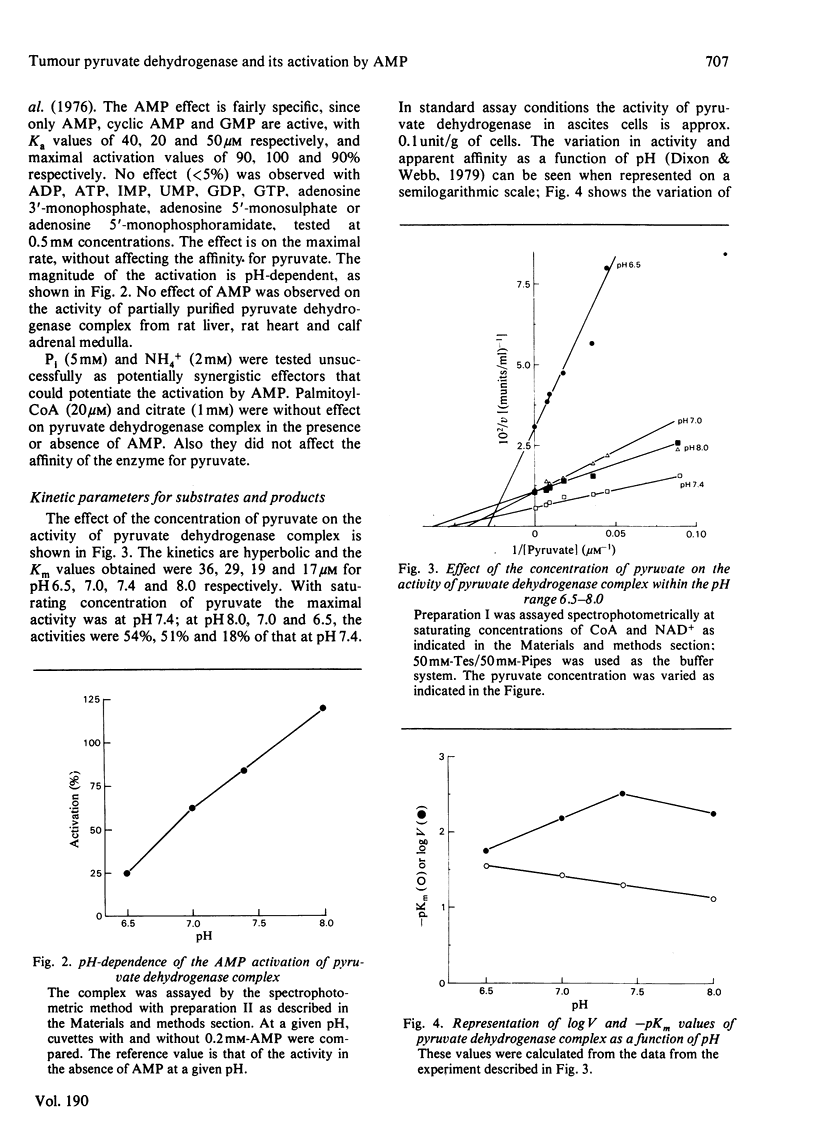
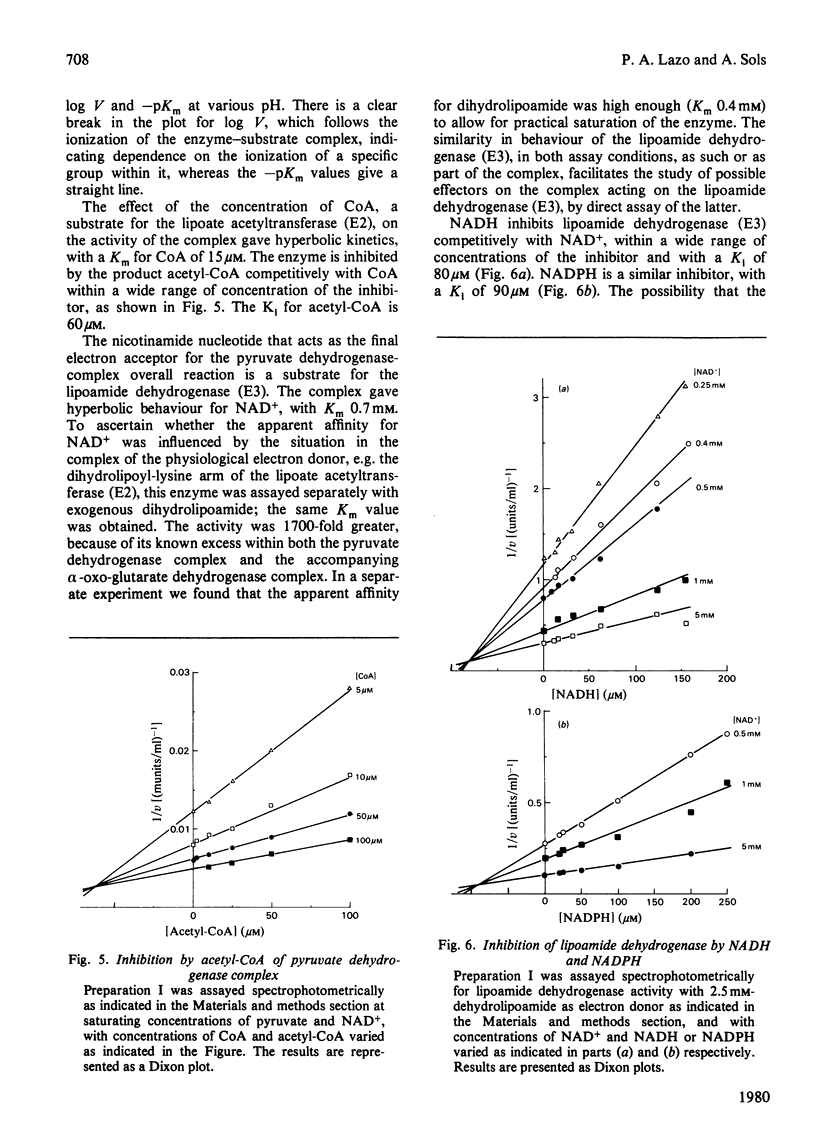
1. AMP is an activator of the pyruvate dehydrogenase complex of the Ehrlich--Lettré ascites tumour, increasing its V up to 2-fold, with Ka of 40 microM at pH 7.4. This activation appears to be an allosteric effect on the decarboxylase subunit of the complex. 2. The pyruvate dehydrogenase complex has a Km for pyruvate within the range 17--36 microM depending on the pH, the optimum pH being approx. 7.4, with a V of approx. 0.1 unit/g of cells. The rate-limiting step is dependent on the transformation of the enzyme--substrate complex. The Km for CoA is 15 microM. The Km for NAD+ is 0.7 mM for both the complex and the lipoamide dehydrogenase. The complex is inhibited by acetyl-CoA competitively with CoA; the Ki is 60 microM. The lipoamide dehydrogenase is inhibited by NADH and NADPH competitively with NAD+, with Ki values of 80 and 90 microM respectively. In the reverse reaction the Km values for NADH and NADPH are essentially equal to their Ki values for the forward reaction, the V for the latter being 0.09 of that of the former. Hence the reaction rate of the complex in vivo is likely to be markedly affected by feedback isosteric inhibition by reduced nicotinamide nucleotides and possibly acetyl-CoA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg J. J., Olson M. S. Regulation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. J Biol Chem. 1976 Mar 10;251(5):1364–1370. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bresters T. W., de Kok A., Veeger C. The pyruvate-dehydrogenase complex from Azotobacter vinelandii. 2. Regulation of the activity. Eur J Biochem. 1975 Nov 15;59(2):347–353. doi: 10.1111/j.1432-1033.1975.tb02461.x. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. An amino acid sequence in the active site of lipoamide dehydrogenase from pig heart. Biochem J. 1974 Mar;137(3):505–512. doi: 10.1042/bj1370505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. An amino acid sequence in the active site of lipoamide dehydrogenase from the 2-oxoglutarate dehydrogenase complex of E. coli (Crookes strain). FEBS Lett. 1972 Oct 1;26(1):221–224. doi: 10.1016/0014-5793(72)80577-5. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Perham R. N. Selective inactivation of the transacylase components of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem J. 1976 May 1;155(2):419–427. doi: 10.1042/bj1550419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Roche T. E. A unifying mechanism for stimulation of mammalian pyruvate dehydrogenase(a) kinase by reduced nicotinamide adenine dinucleotide, dihydrolipoamide, acetyl coenzyme A, or pyruvate. J Biol Chem. 1978 Jan 25;253(2):496–503. [PubMed] [Google Scholar]
- Eboli M. L., Paradies G., Galeotti T., Papa S. Pyruvate transport in tumour-cell mitochondria. Biochim Biophys Acta. 1977 Apr 11;460(1):183–187. doi: 10.1016/0005-2728(77)90166-9. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. The intracellular distribution of pyridine nucleotides in rat liver. Exp Cell Res. 1956 Aug;11(1):234–236. doi: 10.1016/0014-4827(56)90214-2. [DOI] [PubMed] [Google Scholar]
- HATHAWAY J. A., ATKINSON D. E. THE EFFECT OF ADENYLIC ACID ON YEAST NICOTINAMIDE ADENINE DINUCLEOTIDE ISOCITRATE DEHYDROGENASE, A POSSIBLE METABOLIC CONTROL MECHANISM. J Biol Chem. 1963 Aug;238:2875–2881. [PubMed] [Google Scholar]
- Hansford R. G., Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978 Nov;191(1):65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Johnson M. J. THE ROLE OF AEROBIC PHOSPHORYLATION IN THE PASTEUR EFFECT. Science. 1941 Aug 29;94(2435):200–202. doi: 10.1126/science.94.2435.200. [DOI] [PubMed] [Google Scholar]
- Marco R., Pestaña A., Sebastian J., Sols A. Oxaloacetate metabolic crossroads in liver. Enzyme compartmentation and regulation of gluconeogenesis. Mol Cell Biochem. 1974 Mar 8;3(1):53–70. doi: 10.1007/BF01660077. [DOI] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. 31P nuclear magnetic resonance studies of Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):87–91. doi: 10.1073/pnas.74.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. S., Dennis S. C., DeBuysere M. S., Padma A. The regulation of pyruvate dehydrogenase in the isolated perfused rat heart. J Biol Chem. 1978 Oct 25;253(20):7369–7375. [PubMed] [Google Scholar]
- Olson M. S., Dennis S. C., Routh C. A., Debuysere M. S. The regulation of pyruvate dehydrogenase by fatty acids in isolated rabbit heart mitochondria. Arch Biochem Biophys. 1978 Apr 15;187(1):121–131. doi: 10.1016/0003-9861(78)90014-0. [DOI] [PubMed] [Google Scholar]
- Perham R. N. Self-assembly of biological macromolecules. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):123–136. doi: 10.1098/rstb.1975.0075. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. 8. Comparison of dihydrolipoyl dehydrogenases from pyruvate and alpha-ketoglutarate dehydrogenase complexes of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1126–1130. doi: 10.1073/pnas.58.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postius S. Continuous measurement of metabolically produced 14CO2 within the range of minutes. Anal Biochem. 1978 Oct 15;90(2):534–542. doi: 10.1016/0003-2697(78)90147-1. [DOI] [PubMed] [Google Scholar]
- REED L. J., KOIKE M., LEVITCH M. E., LEACH F. R. Studies on the nature and reactions of protein-bound lipoic acid. J Biol Chem. 1958 May;232(1):143–158. [PubMed] [Google Scholar]
- Roche T. E., Cate R. L. Purification of porcine liver pyruvate dehydrogenase complex and characterization of its catalytic and regulatory properties. Arch Biochem Biophys. 1977 Oct;183(2):664–677. doi: 10.1016/0003-9861(77)90400-3. [DOI] [PubMed] [Google Scholar]
- Roche T. E., Reed L. J. Function of the nonidentical subunits of mammalian pyruvate dehydrogenase. Biochem Biophys Res Commun. 1972 Aug 21;48(4):840–846. doi: 10.1016/0006-291x(72)90684-5. [DOI] [PubMed] [Google Scholar]
- Sauer L. A. Control of adenosine monophosphate catabolism in mouse ascites tumor cells. Cancer Res. 1978 Apr;38(4):1057–1063. [PubMed] [Google Scholar]
- Siess E. A., Nimmannit S., Wieland O. H. Kinetic and regulatory properties of pyruvate dehydrogenase from Ehrlich ascites tumor cells. Cancer Res. 1976 Jan;36(1):55–59. [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- WEINHOUSE S. On respiratory impairment in cancer cells. Science. 1956 Aug 10;124(3215):267–269. doi: 10.1126/science.124.3215.267. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Cooper R. H., Denton R. M., Bridges B. J., Randle P. J. The elementary reactions of the pig heart pyruvate dehydrogenase complex. A study of the inhibition by phosphorylation. Biochem J. 1976 Jul 1;157(1):41–67. doi: 10.1042/bj1570041. [DOI] [PMC free article] [PubMed] [Google Scholar]


