Abstract
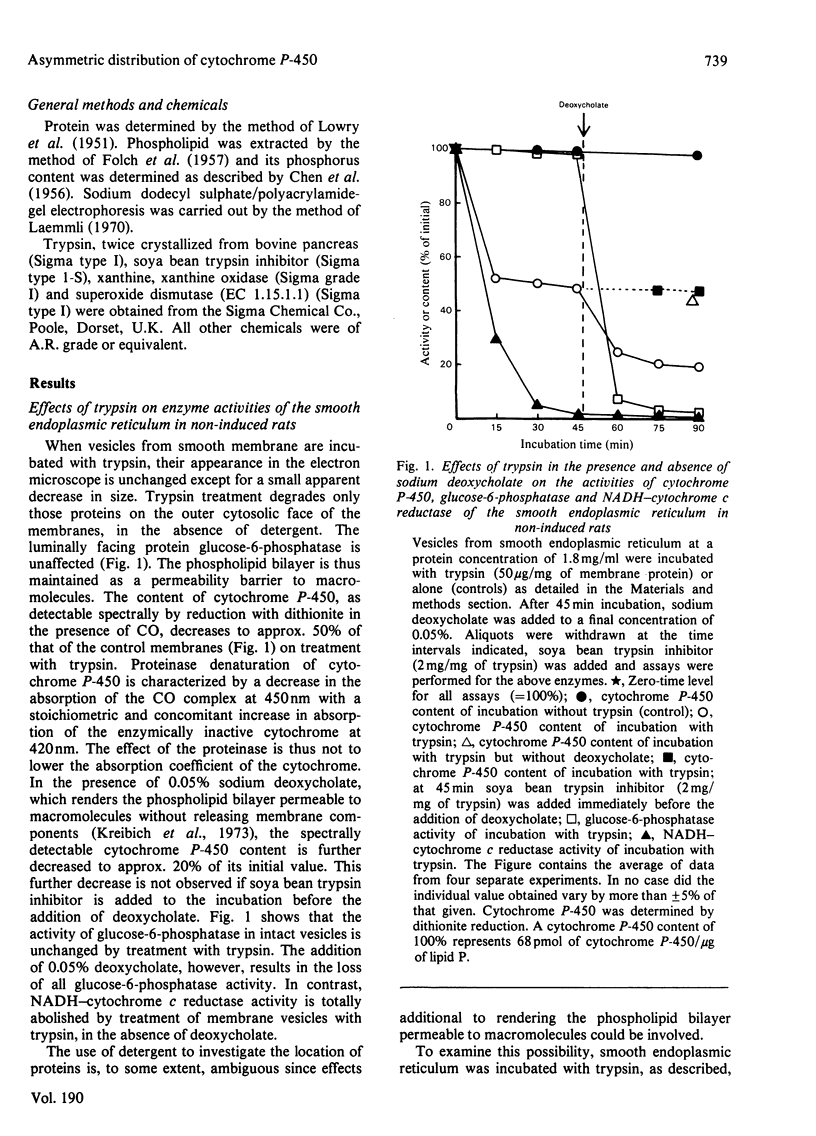
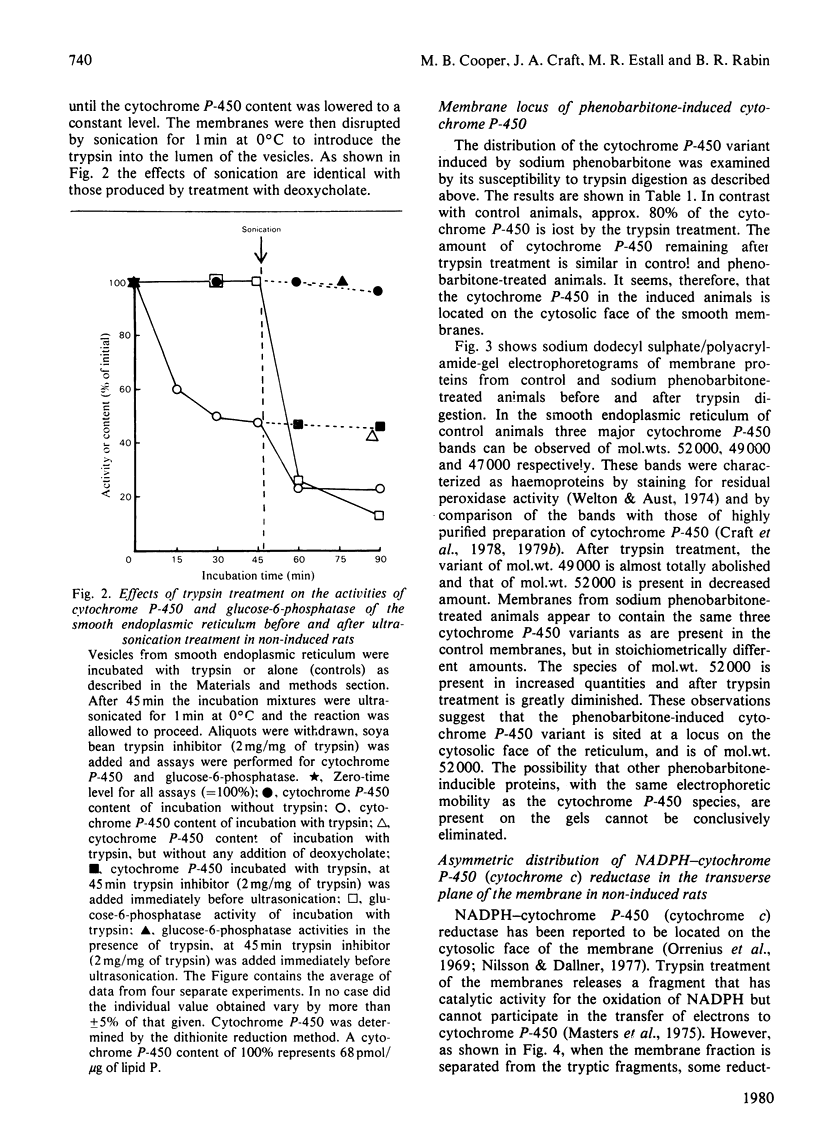
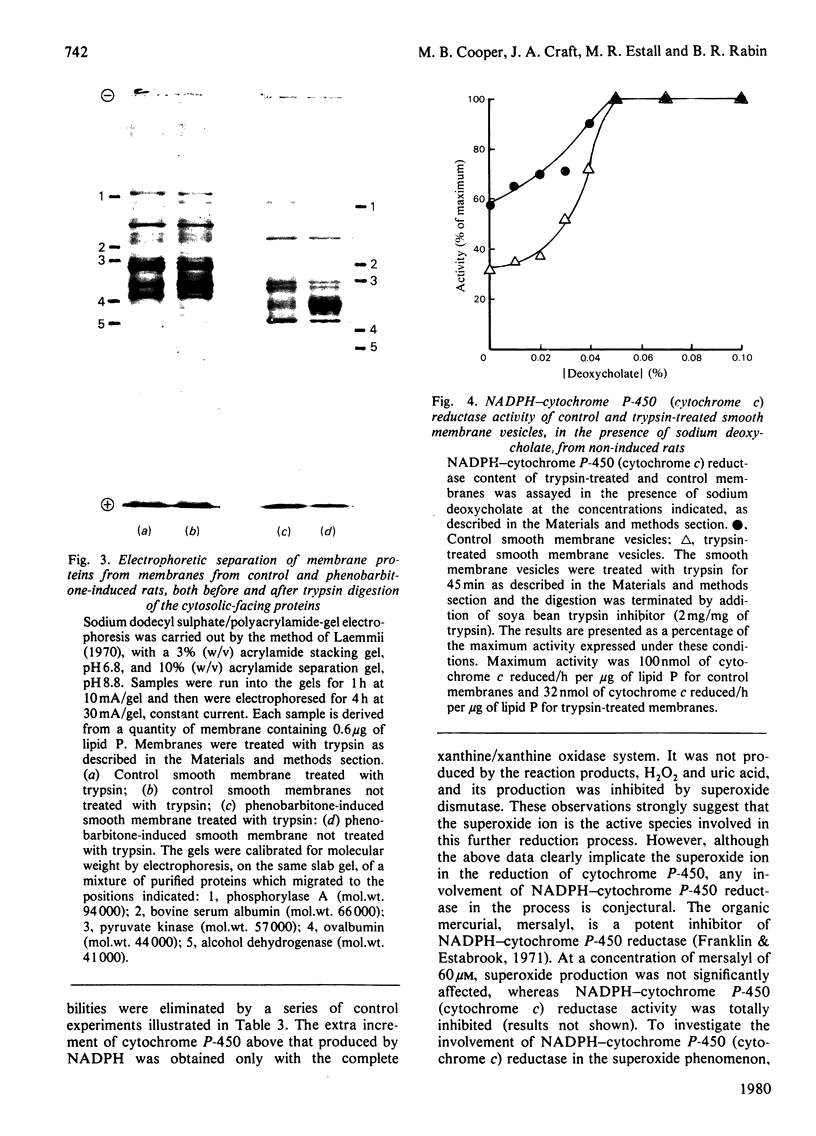
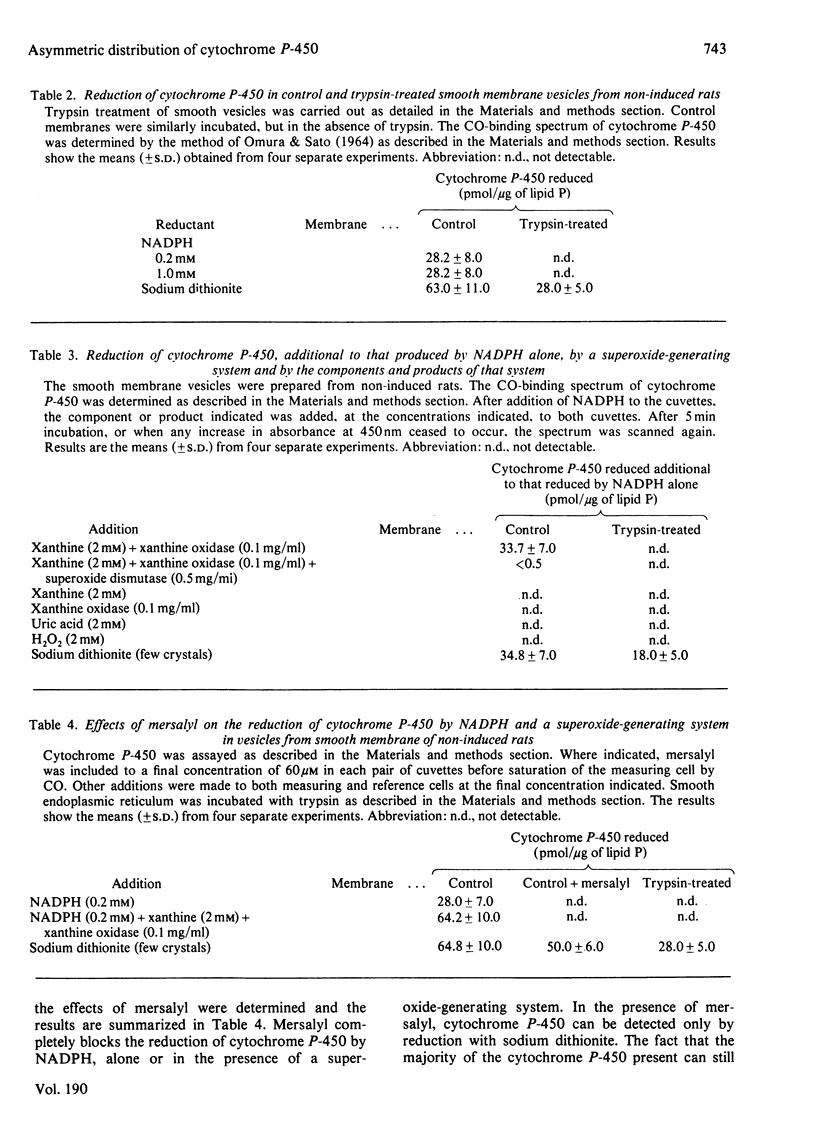
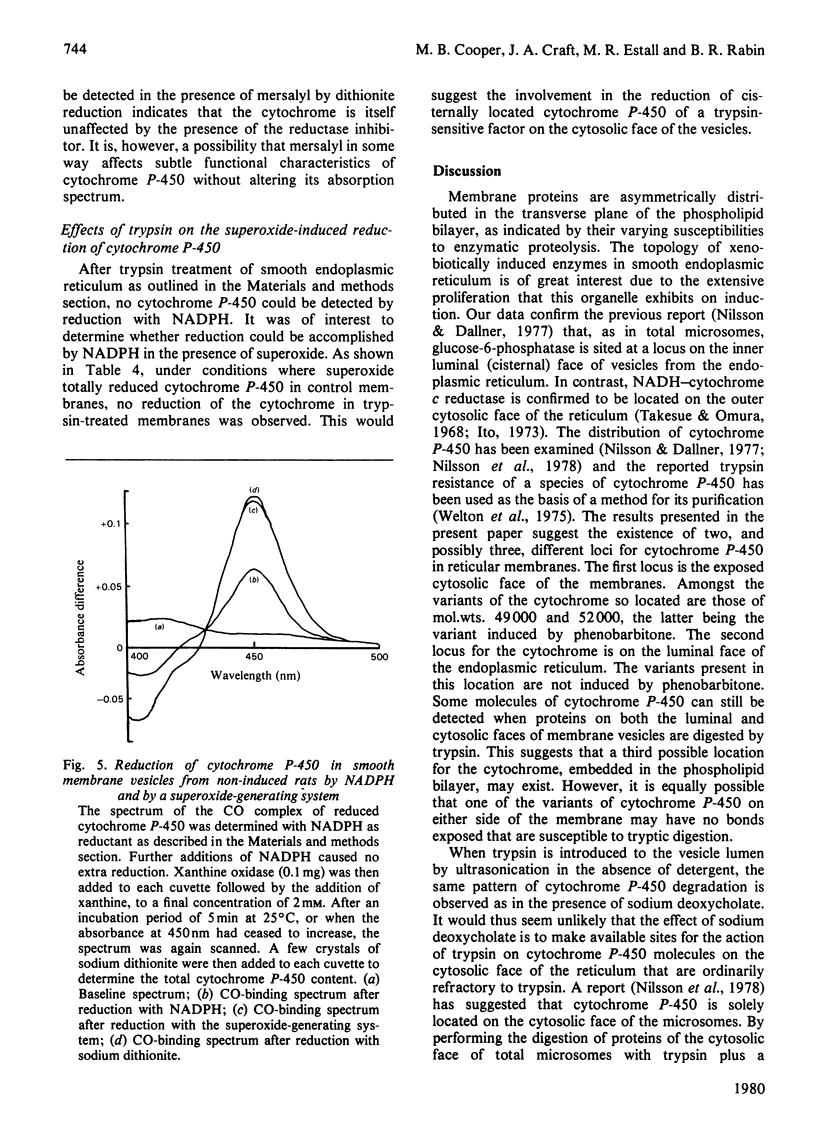
1. The topography of cytochrome P-450 in vesicles from smooth endoplasmic reticulum of rat liver has been examined. Approx. 50% of the cytochrome is directly accessible to the action of trypsin in intact vesicles whereas the remainder is inaccessible and partitioned between luminal-facing or phospholipid-embedded loci. Analysis by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis reveals three major species of the cytochrome. Of these, the variant with a mol.wt. of 52000 is induced by phenobarbitone and this species is susceptible to trypsin. 2. After trypsin treatment of smooth membrane, some NADPH–cytochrome P-450 (cytochrome c) reductase activity remains and this remaining activity is enhanced by treatment with 0.05% deoxycholate, which renders the membranes permeable to macromolecules. In non-trypsin-treated control membranes the reductase activity is increased to a similar extent. These observations suggest an asymmetric distribution of NADPH–cytochrome P-450 (cytochrome c) reductase in the membrane. 3. As compared with dithionite, NADPH reduces only 44% of the cytochrome P-450 present in intact membranes. After tryptic digestion, none of the remaining cytochrome P-450 is reducible by NADPH. 4. In the presence of both a superoxide-generating system (xanthine plus xanthine oxidase) and NADPH, all the cytochrome P-450 in intact membrane (as judged by dithionite reducibility) is reduced. The cytochrome P-450 remaining after trypsin treatment of smooth vesicles cannot be reduced by this method. 5. The superoxide-dependent reduction of cytochrome P-450 is prevented by treatment of the membranes with mersalyl, which inhibits NADPH–cytochrome P-450 (cytochrome c) reductase. Thus the effect of superoxide may involve NADPH–cytochrome P-450 reductase and cytosolically orientated membrane factor(s).
Full text
PDF




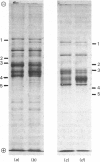
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Roerig D. L., Pederson T. C. Evidence for superoxide generation by NADPH-cytochrome c reductase of rat liver microsomes. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1133–1137. doi: 10.1016/0006-291x(72)90952-7. [DOI] [PubMed] [Google Scholar]
- Bergman A., Dallner G. Properties of a rat liver smooth microsomal subfraction not aggregated by Mg2+. Life Sci. 1976 May 15;18(10):1083–1090. doi: 10.1016/0024-3205(76)90142-9. [DOI] [PubMed] [Google Scholar]
- Blyth C. A., Freedman R. B., Rabin B. R. The effects of aflatoxin B1 on the sex-specific binding of steroid hormones to microsomal membranes of rat liver. Eur J Biochem. 1971 Jun 29;20(4):580–586. doi: 10.1111/j.1432-1033.1971.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Craft J. A., Cooper M. B., Estall M. R., Rabin B. R. The biosynthesis of cytochrome P450 by rough endoplasmic reticulum in vitro. A significant proportion of newly-biosynthesised cytochrome P450 is resistant to proteolytic digestion in intact vesicles. FEBS Lett. 1979 Feb 15;98(2):403–407. doi: 10.1016/0014-5793(79)80227-6. [DOI] [PubMed] [Google Scholar]
- Craft J. A., Cooper M. B., Estall M. R., Rees D. E., Rabin B. R. The role of components of the endoplasmic reticulum in the biosynthesis of cytochrome P-450. Eur J Biochem. 1979 May 15;96(2):379–391. doi: 10.1111/j.1432-1033.1979.tb13050.x. [DOI] [PubMed] [Google Scholar]
- Craft J. A., Cooper M. B., Rabin B. R. The biosynthesis of cytochrome P-450 in vitro. FEBS Lett. 1978 Apr 1;88(1):62–66. doi: 10.1016/0014-5793(78)80607-3. [DOI] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. II. Synthesis of constitutive microsomal enzymes in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):97–117. doi: 10.1083/jcb.30.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L., Orrenius S. Substrate-induced synthesis of the hydroxylating enzyme system of liver microsomes. Fed Proc. 1965 Sep-Oct;24(5):1190–1199. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRIDOVICH I. Competitive inhibition by myoglobin of the reduction of cytochrome c by xanthine oxidase. J Biol Chem. 1962 Feb;237:584–586. [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Xanthine oxidase. IV. Participation of iron in internal electron transport. J Biol Chem. 1958 Dec;233(6):1581–1585. [PubMed] [Google Scholar]
- Franklin M. R., Estabrook R. W. On the inhibitory action of mersalyl on microsomal drug oxidation: a rigid organization of the electron transport chain. Arch Biochem Biophys. 1971 Mar;143(1):318–329. doi: 10.1016/0003-9861(71)90213-x. [DOI] [PubMed] [Google Scholar]
- Haugen D. A., Coon M. J. Induction of multiple forms of mouse liver cytochrome P-450. Evidence for genetically controlled de novo protein synthesis in response to treatment with beta-naphthoflavone or phenobarbital. J Biol Chem. 1976 Mar 25;251(6):1817–1827. [PubMed] [Google Scholar]
- Haugen D. A., van der Hoeven T. A., Coon M. J. Purified liver microsomal cytochrome P-450. Separation and characterization of multiple forms. J Biol Chem. 1975 May 10;250(9):3567–3570. [PubMed] [Google Scholar]
- Ito A. Evidence obtained by cathepsin digestion of microsomes for the assembly of cytochrome b5 and its reductase in the membrane. J Biochem. 1974 Apr;75(4):787–793. doi: 10.1093/oxfordjournals.jbchem.a130451. [DOI] [PubMed] [Google Scholar]
- Ito A., Sato R. Proteolytic microdissection of smooth-surfaced vesicles of liver microsomes. J Cell Biol. 1969 Jan;40(1):179–189. doi: 10.1083/jcb.40.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Debey P., Sabatini D. D. Selective release of content from microsomal vesicles without membrane disassembly. I. Permeability changes induced by low detergent concentrations. J Cell Biol. 1973 Aug;58(2):436–462. doi: 10.1083/jcb.58.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntzman R. Drugs and enzyme induction. Annu Rev Pharmacol. 1969;9:21–36. doi: 10.1146/annurev.pa.09.040169.000321. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Junk K. W., Coon M. J. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969 Jul 10;244(13):3714–3721. [PubMed] [Google Scholar]
- MAZUR A., GREEN S., SHORR E. The oxidation of adrenaline by ferritin iron and hydrogen peroxide. J Biol Chem. 1956 May;220(1):227–235. [PubMed] [Google Scholar]
- Master B. S., Prough R. A., Kamin H. Properties of the stable aerobic and anaerobic half-reduced states of NADPH-cytochrome c reductase. Biochemistry. 1975 Feb 11;14(3):607–613. doi: 10.1021/bi00674a022. [DOI] [PubMed] [Google Scholar]
- Masters B. S., Baron J., Taylor W. E., Isaacson E. L., LoSpalluto J. Immunochemical studies on electron transport chains involving cytochrome P-450. I. Effects of antibodies to pig liver microsomal reduced triphosphopyridine nucleotide-cytochrome c reductase and the non-heme iron protein from bovine adrenocortical mitochondria. J Biol Chem. 1971 Jul 10;246(13):4143–4150. [PubMed] [Google Scholar]
- Nilsson O. S., Dallner G. Enzyme and phospholipid asymmetry in liver microsomal membranes. J Cell Biol. 1977 Mar;72(3):568–583. doi: 10.1083/jcb.72.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O. S., DePierre J. W., Dallner G. Investigation of the transverse topology of the microsomal membrane using combinations of proteases and the non-penetrating reagent diazobenzene sulfonate. Biochim Biophys Acta. 1978 Jul 20;511(1):93–104. doi: 10.1016/0005-2736(78)90067-6. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Orrenius S., Berg A., Ernster L. Effects ofrypsin on the electron transport systems of liver microsomes. Eur J Biochem. 1969 Nov;11(1):193–200. doi: 10.1111/j.1432-1033.1969.tb00760.x. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Rees D. E. The mechanism of induction of the microsomal drug hydroxylating system in rat liver by phenobarbital. Gen Pharmacol. 1979;10(5):341–350. doi: 10.1016/0306-3623(79)90068-5. [DOI] [PubMed] [Google Scholar]
- Strobel H. W., Coon M. J. Effect of superoxide generation and dismutation on hydroxylation reactions catalyzed by liver microsomal cytochrome P-450. J Biol Chem. 1971 Dec 25;246(24):7826–7829. [PubMed] [Google Scholar]
- Strobel H. W., Lu A. Y., Heidema J., Coon M. J. Phosphatidylcholine requirement in the enzymatic reduction of hemoprotein P-450 and in fatty acid, hydrocarbon, and drug hydroxylation. J Biol Chem. 1970 Sep 25;245(18):4851–4854. [PubMed] [Google Scholar]
- Takesue S., Omura T. Enzymatic solubilization of microsomal NADH-cytochrome b5 reductase by lysosomes. Biochem Biophys Res Commun. 1968 Mar 27;30(6):723–729. doi: 10.1016/0006-291x(68)90573-1. [DOI] [PubMed] [Google Scholar]
- Vermilion J. L., Coon M. J. Highly purified detergent-solubilized NADPH-cytochrome P-450 reductase from phenobarbital-induced rat liver microsomes. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1315–1322. doi: 10.1016/0006-291x(74)90341-6. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Aust S. D. Multiplicity of cytochrome P450 hemoproteins in rat liver microsomes. Biochem Biophys Res Commun. 1974 Feb 27;56(4):898–906. doi: 10.1016/s0006-291x(74)80273-1. [DOI] [PubMed] [Google Scholar]
- Welton A. F., O'Neal F. O., Chaney L. C., Aust S. D. Multiplicity of cytochrome P-450 hemoproteins in rat liver microsomes. Preparation and specificity of an antibody to the hemoprotein induced by phenobarbital. J Biol Chem. 1975 Jul 25;250(14):5631–5639. [PubMed] [Google Scholar]