Abstract
In this study, the conversion of bones (waste of food industry) into bone char is described. The presence of calcium phosphate and graphitic carbon gives bone char unique properties, with different possible uses. The catalytic behavior of bone char modified with chlorosulfonic acid is tested as reusable and eco-friendly solid acid biocatalyst in synthesis of pyrimidine-5-carbonitrile derivatives. The catalyst properties were characterized by different techniques such as FT-IR, BET, SEM, TEM, TGA, EDS and XRD. The bone char catalyst can be reused several times without reducing catalyst efficiency. In addition to the various reported benefits, the first use of bone char as a catalyst support for organic synthesis is an important novelty of this study.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82040-3.
Keywords: Bone char, Pyrimidine-5-carbonitrile, Nano catalyst, Green catalyst, Hydroxy apatite
Subject terms: Catalysis, Organic chemistry
Introduction
Materials with ordered porous properties at the nanoscale have important uses in different fields such as optics, drug delivery systems, catalysis, coatings, cosmetics, diagnostics, bio separation, nanotechnology, and air separation. Most nano-porous materials fall into three broad categories: micro, meso, and macro1. Mesoporous materials are of vast attention in the fields of science and technology due to their many advantages, including large surface area and high pore volume, greater accessibility, and the ability to adapt and interact with different chemical functions on their surface2,3. Porous carbon materials are known to be very effective adsorbents. In particular, those obtained from biological waste have been widely studied in recent years because of their outstanding effectiveness in removing organic pollutants and heavy metals4–8. In this context, low-cost carbon materials derived from natural-based based have particular interest.
Biochar is a heterogeneous carbon material consisting of some functional groups of many levels, which is produced by the thermal conversion of various types of waste. Biochar is usually a carbon-rich product obtained when different biomass such as wood, manure, leaves, sludge, municipal sewage, etc. is heated in a closed system with slight or no oxygen9. also, Animal bone char is another produce of carbon-containing material that can be produced under similar controlled thermal conditions to produce a phosphorus-rich product10. Bone char is a solid material that is produced from the pyrolysis of animal bones and is usually used as a decolorizing adsorbent in refined sugar. During this process, commonly the bone is heated in an inert or oxidized atmosphere at a temperature between 400 and 600 0C, for control of the quality and efficiency of the product11.
Bone char is usually composed of two materials carbon and hydroxyapatite (HAP) [Ca10 (PO4)6 (OH)2]12. It is constituted by calcium hydroxyapatite (70–76%), carbon (9–11%), and calcium carbonate (7–9%) and has a surface area of approximately 100 m2 g− 1 with a structure composed exclusively of mesoporous13–15. Further pore diameter detection shows that the pore sizes of bone char range from 1.7 to 75 nm, including major mesopores (2 to 50 nm) and a small number of pores with a diameter of 50 nm (macropores)16. Bone char has attracted attention due to its cheapness, ease of production, and biocompatibility17. Various reactions such as degradation, condensation, and hydrogen removal are possible and cause “accumulation” of these materials on the surface of hydroxyapatite crystals18. Among the materials utilized as adsorbents, usually bone char has been widely used to remove various pollutants in drinking water19,20.
Heterocyclic compounds are of biological attentiveness due to their potential chemical and physical properties21. Among these, pyrimidine derivatives have attracted considerable attention for their pharmacological properties such as bactericidal, fungicidal22, analgesic23, anti-inflammatory24, anti-cancer25, antioxidant26, and anti-HIV27. The synthetic diversification of pyrimidine allows the production of a variety of derivatives, including analogs resulting from aryl ring substitution, pyrimidine nitrogen derivatization, and carbon substitution at the 2, 4, 5, and 6 positions27. Although some routes of pyrimidine synthesis have been known for a long ago, the development of other more economical routes is of substantial importance28,–30.
In continuation of our investigation on biowaste-based catalysis systems31–35, In this work, we develop an efficient method for one-pot synthesis of pyrimidine-5-carbonitrile derivatives by condensation of three components including aromatic aldehydes, malononitrile, and thiourea or urea in the presence of bone char-nPrN-SO3H as a recyclable nanocatalyst in solvent-free conditions at 80 °C. The results obtained from the study of the synthesis of pyrimidine-5-carbonitrile showed excellent yield and reduced reaction time to less than a few minutes.
Materials and methods
Materials and methods
The chemicals used in this research were acquired from Sigma-Aldrich and employed without undergoing additional purification. The XRD pattern was carried out using a PANalytical. Data were recorded in the diffractometer with CuKα radiations in diverse angle range 2 ≤ °2θ ≤ 90, operating at 40kv and 30 mA. Instruments used in Brunauer-Emmett-Teller have this character with Belsorp company, Japan. In order to observe changes in the functional groups, the FTIR spectra of the materials were collected using a KBr pellet and a Nicolet MAGNA-IR 550 spectrometer (Madison, WI, USA). The transmission electron microscopy (TEM) technique was employed with a TEM Philips EM 208 S instrument, operating at an accelerating voltage of 100 kV, to analyze the morphology and particle size. X-ray energy dispersive spectroscopy (EDX) was employed to determine the elemental composition of a sample. Thermogravimetric analyses (TGA) of the samples were recorded using a Shimadzu PL-STA 1500 device in the temperature range 30–800 °C used to study the thermal behavior of materials. To monitor the temperature of a synthesized product the melting points were measured using the electrothermal IA9200 apparatus. The reaction progress and evaluation of substrate purity were monitored using thin-layer chromatography (TLC) on silica gel SILG/UV 254 and 365 plates.
Preparation BC
first bovine bones were crushed into 1–3 cm pieces, then washed three times in hot deionized water for 4 h to remove fat and protein residues, the achieve bones pieces were dried overnight at 110 °C. Bone pyrolysis was performed in an air (oxygen)-free furnace at 450 °C for 4.5 h with a heating rate of 10 °C/min to produce bone char. The bone char was allowed to cool to room temperature, then crushed and sieved to obtain particles with a diameter of 0.2 mm. Finally, the obtained sample is stored in a sealed container.
Functionalization of bone char surfaces with 3-aminopropyltriethoxysilane
Firstly 6 gr, 3-aminopropyltriethoxysilane was added in 50 ml of the ethanol/water (9/1 Stir in a 100 ml flask at room temperature (25 ± 2 °C)) with a magnetic stirrer for approximate 30 min. Then 3 g of bone char obtained from the previous step was added to the solution and stirred at reflux condition for 24 h. Finally, the functionalization of bone char was washed for several times with water (H2O) and ethanol (EtOH) and dried at room temperature.
Bone char-nPrN-SO3H
Accurate 0.5 g of functionalization of bone char powder was dispersed in sonicated in 20 mL of dichloromethane) CH2Cl2) for 20 min. Then 2 ml of chlorosulfonic acid (ClSO3H) was added dropwise and stirred in an ice bath for 3 h. Finally, the synthesized nano catalyst was washed for several time with water (H2O) and ethanol (EtOH) and dried at room temperature. Also, to determine the amount of acid in catalyst according to the literature,13 the 0.1 g of catalyst was added to an aqueous NaCl solution (1 mol/L, 10 mL) with an initial pH 7.1. The mixture was stirred for 30 min until the pH of solution decreased to 3.1 that indicating an ion exchange between sulfamic acid protons and sodium ions and this is equal to a loading of 0.079 mmol.g− 1 of sulfamic acid group.
General procedure for the synthesis of pyrimidine-5-carbonitrile derivatives
A mixture of aldehyde derivatives (1 mol), urea/thiourea (1.8 mol), malononitrile (1.2 mol) and bone char-nPrN-SO3H (0.4 mol%) were placed in a round-bottom flask in solvent-free conditions at 80 °C, then mixture was stirred for the appropriate time. After the reaction was completed (by TLC thin layer chromatography), distilled water was added to the reaction mixture and cooled to room temperature. The resulting precipitate was separated by filtration and washed with hot ethanol (3 × 2 ml). The precipitate was then recrystallized from ethanol giving the pure product.
Selected NMR data
6-Amino-4-(4-boromophenyl)-5-cyano-2-hydroxypyrimidine: 1H NMR (250 MHz, DMSO-d6) 8.62 (s, 2 H), 8.03 (d, J = 8.5 Hz, 2 H), 7.90 (s, 1H), 7.70 (d, J = 8.6 Hz, 2 H).13C NMR (63 MHz, DMSO-d6) δ 157.2, 139.2, 135.8, 131.4, 130.5, 128.8, 113.8, 112.7, 87.5. IR (KBr): 3422 (cm− 1) (broad, OH), 3091 (cm− 1) (NH2), 2227 (cm− 1) and 1639 (cm− 1) (CN).
6-Amino-4-(2,4-dichlorophenyl)-5-cyano-2-hydroxypyrimidine: 1H NMR (250 MHz, DMSO-d6) δ 8.51 (s, 2 H), 8.19–7.54 (m, 4 H). 13C NMR (63 MHz, DMSO-d6) δ 182.3, 160.7, 134.8, 134.4, 133.1, 132.6, 130.8, 128.8, 114.5, 113.4, 82.8. IR (KBr): 3422 (cm− 1) (broad, OH), 3102 (cm− 1) (NH2), 2229 (cm− 1) and 1639 (cm− 1) (CN).
6-Amino-5-Cyano-4-(4-boromo)-Phenyl-2-MercaptoPyrimidine: 1H NMR (250 MHz, DMSO-d6) δ 8.50 (s, 2 H), 7.86 (d, J = 7.4 Hz, 4 H), 2.71 (s, 1H). 13C NMR (63 MHz, DMSO-d6) δ 178.5, 162., 160.7, 133.1, 132.6, 130.8, 128.8, 114.5, 84.5, IR (KBr): 3410 (cm− 1) (NH2), 3032 (cm− 1) (C-H), 2227 (cm− 1) and 1639(cm− 1) (CN).
Result and discussion
Catalyst characterization
To gain insight into the properties of bone char-nPrN-SO3H synthesized as Bronsted acid from cattle bone, FTIR, SEM, TEM, EDX, and XRD analysis was performed and discussed as follows.
SEM analysis
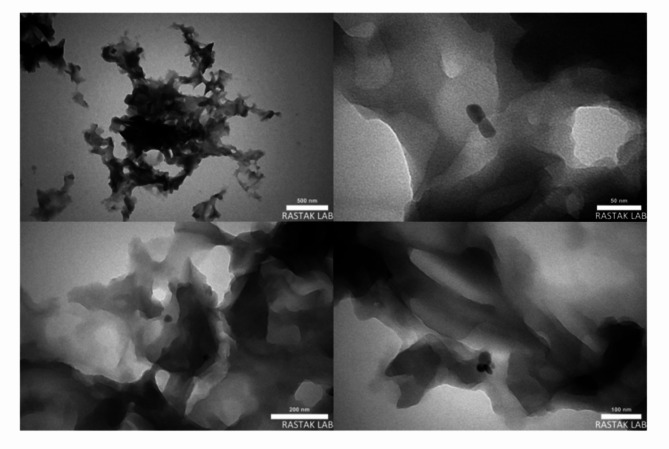
Scanning electron microscopy (SEM) effectively examines the surface structures and morphological characteristics of synthesized catalysts. The SEM image of modification bone char shown in Fig. 1. The modified bone char, used as a Brønsted acid catalyst, was characterized using SEM to assess its morphology and typographical structure. The SEM images of the bone char -nPrN-SO3H catalyst reveal rough and uneven surface structure with irregular flaky particles of varying sizes that are stacked and agglomerated due to their small dimensions. The SEM images in surface display small amount of apparent porosity that confirming their nanoscale size. Also, the transmission electron microscopy (TEM) images show rice and needle-like crystal shapes36. Rice-shaped particles have small diameter and length, while needle-shaped particles have small diameters, longer lengths, and a uniform morphology. The TEM images of bone char -nPrN-SO3H catalyst is shown in Fig. 2.
Fig. 1.
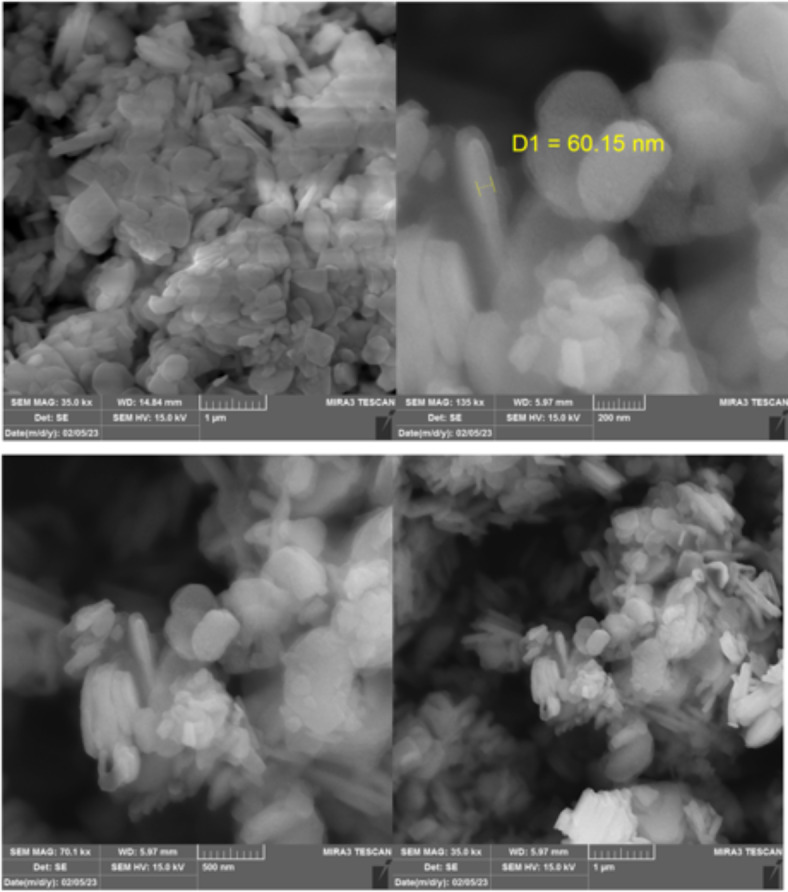
SEM image of modified bone char as Bronsted acid catalysis.
Fig. 2.
TEM image of modified bone char as Bronsted acid catalyst.
XRD analysis
The XRD study was performed on modified bone char as shown in Fig. 3. The pattern obtained corresponds to the standard pattern of hydroxyapatite crystals. Diffraction peaks were observed at 2θ (degree) of 25.4°, 28.6°, 31.3°, 32.0°, 43.3° and 48.7° were appeared in good match with the crystal planes of composition hydroxyapatite (002), (211), (112) and (312), respectively. Sharper peaks observed in the XRD pattern indicate better crystallinity. Also, the peak observed at 25.4º is relates to the c-axis of the hydroxyapatite structure37. The diffraction of plane 002 and 211 results in the highest intensity 25.4º and 31.3º than all other peaks in obtained pattern33. In addition, the dihydroxylation reaction of hydroxyapatite can also cause the peak shift of XRD patterns38.
Fig. 3.
XRD pattern of bone char -nPrN-SO3H catalyst.
BET analysis (surface area and porosity analysis)
BET is the most commonly used method to measure the surface area of a porous compound using N2 gas as an adsorbate on the material surface. The specific surface properties of catalyst obtained at a pyrolysis temperature of 450 °C were determined using N2 adsorption at 77.35 K and a relative pressure range (P/Po) from 0.0 to 1. According to Brunauer-Emmett-Teller (BET), the specific surface area of the modified bone char sample obtained 61.15 m2 /g with a pore volumes 0.69 cm3/g, and average pore diameter 45 nm.
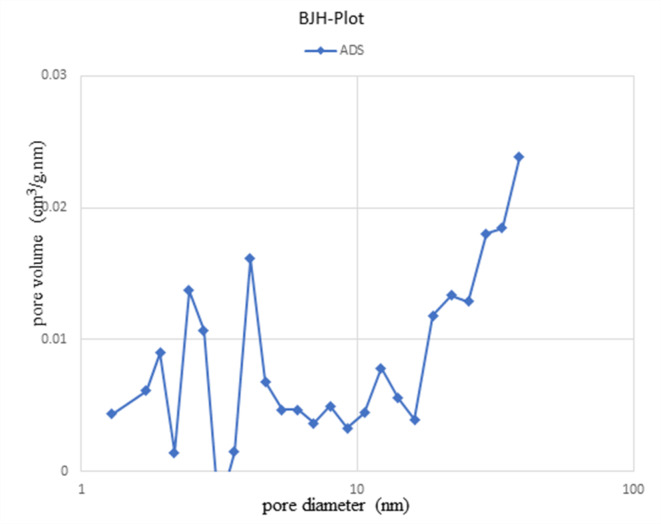
The BJH (Barrett, Joyner, Halenda) method was used to calculating the pore size distribution of the adsorbent. Figure 4 shows the pore size distribution curve of the synthesized bone char sample in this work. The curves show the pore size distribution in the micropore (< 2 nm), mesopore (2–50 nm) and macropore regime (> 50 nm). The analysed sample ranges from 1.2 to 38.52 nm and is mainly distributed in mesoporous mode.
Fig. 4.
BJH adsorption dV/dr Pore volume distribution for bone char-nPrN-SO3H obtained from pyrolyzed of Cattle bones at 450 °C temperatures with residence time 4.5 h.
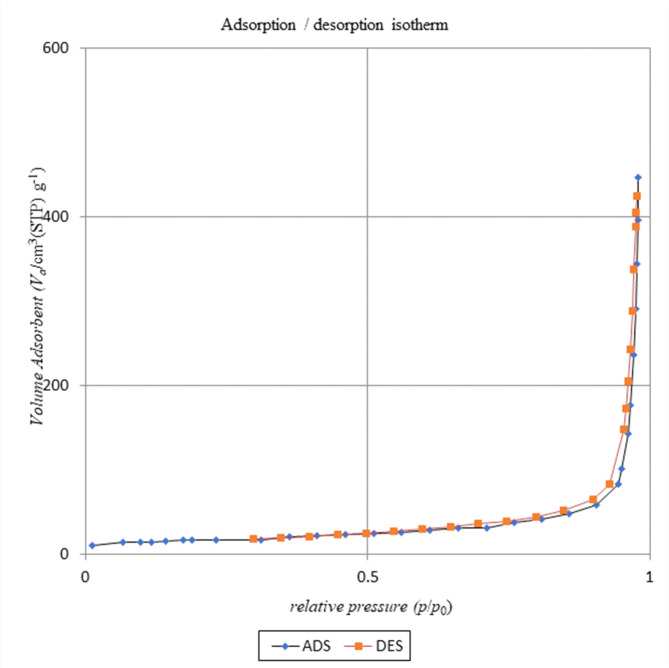
Nitrogen adsorbed at relatively low pressure indicates that thermal decomposition and operating conditions in pyrolysis process may partially cause the formation of micropores, and this hypothesis can be confirmed by the obtained isotherm illustrated in Fig. 5. Table 1 shows a brief comparison from parameter such as surface area and Pore volume in this work with other results available in the literature39.
Fig. 5.
Nitrogen adsorption isotherms for bone char-nPrN-SO3H obtained from pyrolyzed of Cattle bones at 450 °C temperatures with residence time 4.5 h.
Table 1.
Comparison of operating parameters on various types of bones.
| Source | Operating parameters | Surface area (m2/g) |
Pore volume (cm3/g) |
Ref. |
|---|---|---|---|---|
| Cattle bones | Temp-600 °C, Rate of heating-10 °C/min, residence time-2 h, pyrolyzed in a rectangular furnace | 50.4 | 0.305 | 40 |
| Cattle bones | Temp-600 °C, Rate of heating-10 °C/min, residence time-1 h, pyrolyzed in rectangular muffle furnace | 57.9 | 0.293 | 40 |
| Cattle bones | A commercial BC was utilized in this study and was fixed by calcining cattle bones by the APELSA company, Mexico. | 75 | 0.22 | 41 |
| Sheep bones | Temp-900 °C, Rate of heating-10 °C/min, residence time-2 h, pyrolyzed in a rectangular furnace | 38 | 0.16 | 42 |
| Cattle bones | Temp-450 °C, Rate of heating-10 °C/min, residence time-4.5 h, pyrolyzed in a rectangular furnace | 61.15 | 0.69 | This work |
FT-IR spectra
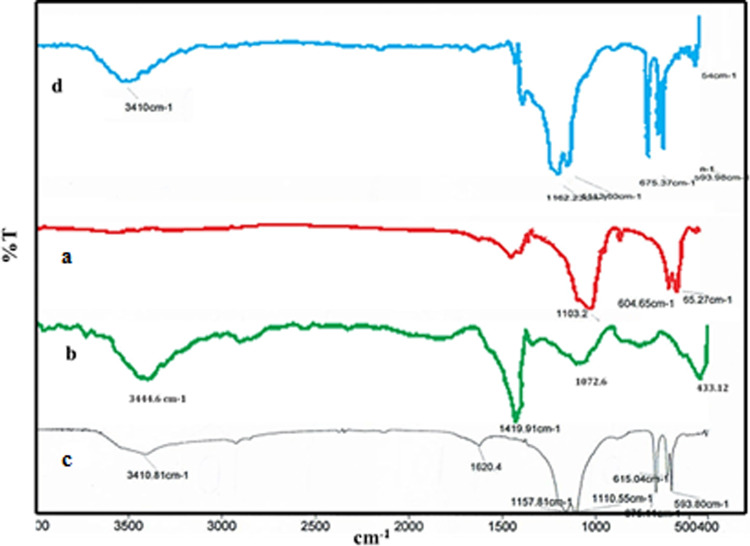
Figure 6 shows the FT-IR spectra of bone char (a), bone char-3-aminopropyl-trietoxysilan (b), bone char-nPrN-SO3H (c) and recovered bone char-nPrN-SO3H (d). In Spectrum (a) the peak observed at 1470 cm− 1correspond to CO3 − 2 group, intensive PO4 − 3 in at 1031 cm− 1 and weak C-H in at 870 cm− 1. The observed peaks at 1031 and 961 cm− 1 are attributed to the vibrational stretching of the PO4 − 3 group. The peaks observed at 604, 565 and 472 cm − 1 correspond to the bending vibrations of PO4 − 3.
Fig. 6.
FT-IR spectrum of modified bone char catalyst.
After functionalization of Bone char with 3-aminopropyl-trietoxysilan (Fig. 7. spectrum (b)) vibration bands of stretch and bend aliphatic CH2 groups, in 1420 cm− 1 and 2988 cm− 1, were observed. Also, peaks were appeared as characteristic Si-O at 1074 cm− 1and as weak O-H at 3411 cm− 1. The peak at 803 cm− 1 may be due to the symmetric starching vibration of Si-O.
Fig. 7.
EDS diagram of Modified bone char catalysis.
The introduction of sulfonic group into the bone char-nPrN-SO3H is evident from the intense peak, in the Fig. 7 spectrum (c), at 1108 cm− 1 and 1161 cm− 1due to the stretching of S = O double bound as well as the broad band at about 3400 cm− 1 assigned to the stretching of the acidic hydroxide (O-H) group.
The FT-IR spectrum of the recovered bone char-nPrN-SO3H was compared with the fresh one shown in the spectrum in Fig. 7(d). The FT-IR spectra of fresh and recycled catalyst are in good agreement. These results indicate the stability of bone char-nPrN-SO3H under reaction conditions.
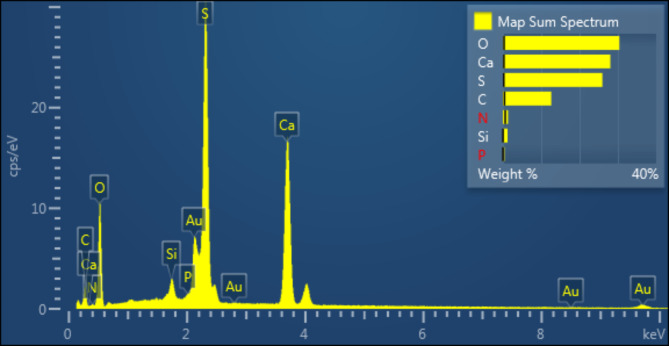
EDS analysis
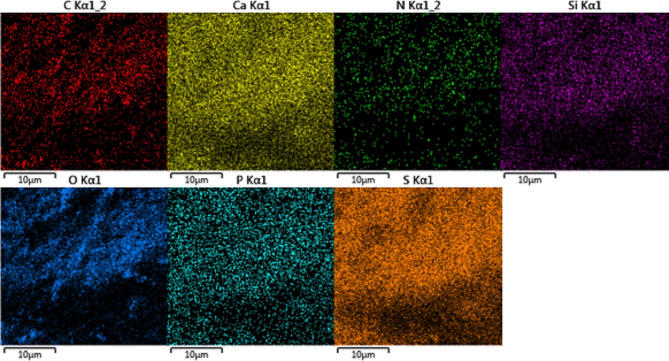
EDS analysis was performed to confirm the elemental content of bone char-nPrN-SO3H obtained at 450 °C (Fig. 7). As seen, the EDS results of modified bone char indicate elements content include calcium (Ca), phosphorus (P), oxygen (O), carbon (C), nitrogen (N), sulfur (S) and silica (Si). Also, Fig. 8 was shown WDX analysis of bone char-nPrN-SO3H as Bronsted acid catalysis. According to Fig. 9, the uniform distribution of oxygen, nitrogen, carbon, silica, calcium, sulfur, and phosphorus was clearly observed in the WDX analysis of Modified Bone Char. By seeing Calcium (Ca), phosphorus (P) and oxygen in SEM-EDS of modified bone char verified the presence of hydroxyapatite composition.
Fig. 8.
Elemental mapping of carbon, calcium, nitrogen, silica, oxygen, phosphorus and sulfur for modified bone char.
Fig. 9.
The TGA diagram of bone char-nPrN-SO3H as Bronsted acid catalysis.
TGA analysis
The TGA diagram of bone char-nPrN-SO3H is shown in Fig. 9. The low weight loss of about 2% at temperatures below 100 °C can be attributed to the evaporation of solvents on the surface of the bone char. Considering that the bone char support is made of bone pyrolysis at a temperature of 450 °C with residence time of 4.5 h, it can be said that the observed weight loss at the temperature 200–600 °C usually due the organic contents (about 11%) were decomposed. This result is strong evidence of the organic layer on the surface of bone char. The TGA curve shows minimal weight loss between 600 and 1000 °C, indicating the stability of the bone char support at high temperatures.
Catalytic activity in synthesis of pyrimidine-5-carbonitrile derivatives
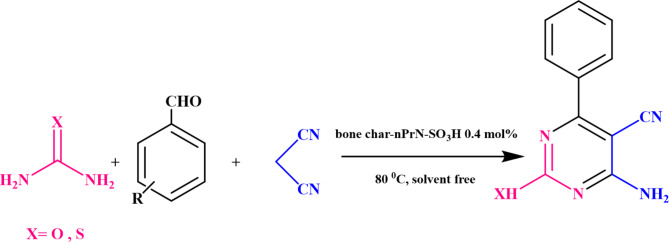
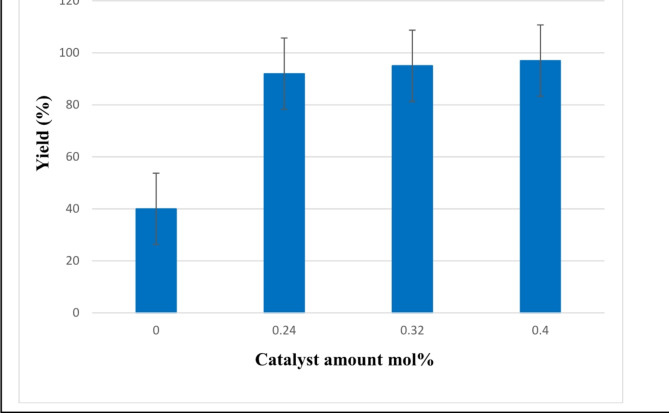
First, the modified bone char catalytic activity was investigated in the synthesis of pyrimidine-5-carbonitrile derivatives (Fig. 10). Then, the reaction parameters such as temperature, the catalyst amount and solvent were optimized in the reaction of 4-chlorobenzaldehyde (1 mol), malononitrile (1.2 mol) and urea (1.8 mol) as a model reaction. The results are summarized in Table 2. It was concluded that solvent-free condition at 80 °C in the presence 0.4 mol% bone char-nPrN-SO3H as the catalyst is the optimized condition for this three-component reaction.
Fig. 10.
Synthesis of pyrimidine-5-carbonitrile derivatives in the presence of bone char-nPrN-SO3H as catalyst in in solvent-free conditions at 80 °C.
Table 2.
Optimization for the synthesis of pyrimidine-5-carbonitrile with 4-chlorobenzaldehyde, urea, malononitrile and bone char-nPrN-SO3H as catalyst.
| Entry | Catalyst (mol%) | Solvent | Temperature (°C) | Time (min) | Yield% |
|---|---|---|---|---|---|
| 1 | 0.00 | - | 80 | 240 | 40 |
| 2 | 0.24 | - | 80 | 65 | 92 |
| 3 | 0.32 | - | 80 | 40 | 95 |
| 4 | 0.4 | - | 80 | 5 | 97 |
| 5 | 0.4 | Water | Reflux | 240 | 75 |
| 6 | 0.4 | Acetonitrile | Reflux | 240 | 50 |
| 7 | 0.4 | n-hexane | Reflux | 240 | 35 |
| 8 | 0.4 | Ethanol | Reflux | 240 | 67 |
| 9 | 0.4 |
Ethanol: water 50:50 |
Reflux | 240 | 71 |
| 10 | 0.4 | - | 30 | 60 | 45 |
| 11 | 0.4 | - | 50 | 35 | 67 |
| 12 | 0.4 | - | 70 | 15 | 85 |
In this study, we described a simple, eco-friendly and economical strategy for the synthesis of pyrimidine-5-carbonitrile. After optimizing the reaction conditions, a highly efficient one-pot synthesis of three-component pyrimidine-5-carbonitrile derivatives were also established using the same method. (Table 3). The reaction of various aromatic aldehydes with malononitrile and urea/thiourea was carried out according to the optimized general experimental procedure. As shown in Table 3, the one-pot three-component reaction are performed efficiently in the presence of presence modified bone char-nPrN-SO3H as Bronsted acid catalysis with various arylaldehydes containing electron-withdrawing and electron-donating groups, such as Cl, Br, Me, NO3, etc., and the yields of most products is over 90%. The great advantage of this system, which distinguishes it from other reported methods, is the very short reaction time. The study of turnover number (TON) and turnover frequency (TOF) in the catalytic system is a very significant area of research which evaluate the efficiency of the catalyst. They refer to the molar number of catalytic sites that converts reactants to products. In this work, TON and TOF were measured for all products and is given in the Table 3. The graphical Error bars is displayed in Fig. 11 to indicate the error bars for different amounts of catalyst.
Table 3.
Synthesis of pyrimidine-5-carbonitrile derivatives in the presence of bone char-nPrN-SO3H as catalyst under solvent-free conditions at 80 °C.
| Entry | R | x | Time (min) | Yield (%) | TON | TOF min− 1 |
|---|---|---|---|---|---|---|
| 1 | H | O | 12 | 91 | 227 | 1137 |
| 2 | 4-Cl | O | 5 | 97 | 242 | 2921 |
| 3 | 4-NO2 | O | 15 | 93 | 232 | 930 |
| 4 | 2,4-Cl2 | O | 4 | 98 | 245 | 3712 |
| 5 | 4-(CH3) | O | 25 | 90 | 225 | 548 |
| 6 | 2-Cl- | O | 9 | 95 | 237 | 1583 |
| 7 | 4-Br | O | 4 | 98 | 245 | 3712 |
| 8 | H | S | 30 | 96 | 245 | 490 |
| 9 | 4-Cl | S | 20 | 95 | 237 | 719 |
| 10 | 4-NO3 | S | 38 | 90 | 225 | 357 |
| 11 | 2,4-Cl2 | S | 23 | 95 | 237 | 625 |
| 12 | 4-(CH3) | S | 40 | 91 | 227 | 344 |
| 13 | 2-Cl | S | 35 | 94 | 235 | 405 |
| 14 | 4-Br | S | 17 | 93 | 232 | 830 |
A mixture of aldehyde derivatives (1 mol), urea/thiourea (1.8 mol), malononitrile (1.2 mol) and bone char-nPrN-SO3H (0.4 mol%) were placed in a round-bottom flask in solvent-free conditions at 80 °C.
Fig. 11.
Catalytic performances synthesis of pyrimidine-5-carbonitrile catalyzed by bone char-nPrN-SO3H at different amounts of catalyst. Error bars indicate the range of data based on repeat experiments.
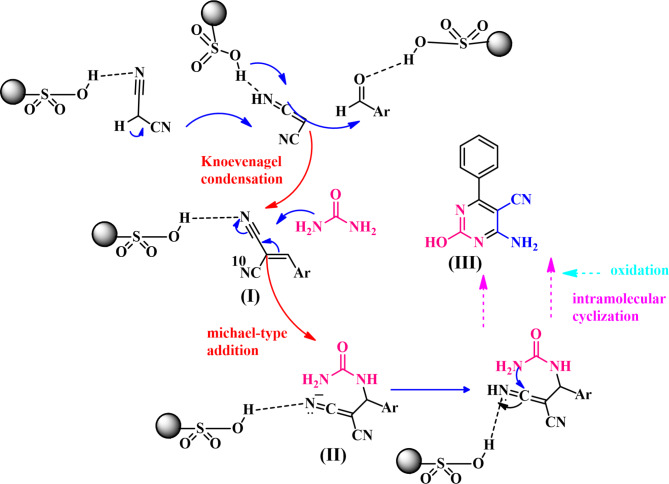
The suggested mechanism for the synthesis of pyrimidine-5-carbonitrile in the presence of bone char-nPrN-SO3H as catalyst, on the basis of the literature43, is proposed in Fig. 12. The condensation of the malononitrile with activated aldehyde by the Knoevenagel reaction give intermediate (I). Then thiourea/urea reacts with (I) by Michael addition to afford the intermediate (II). Finally, cyclodehydration of (II) and tautomerization/aromatization produce pyrimidine-5-carbonitrile (III)40.
Fig. 12.
The possible mechanism for the synthesis of pyrimidine-5-carbonitrile in the presence of bone char-nPrN-SO3H as catalyst.
Recyclability of the catalyst
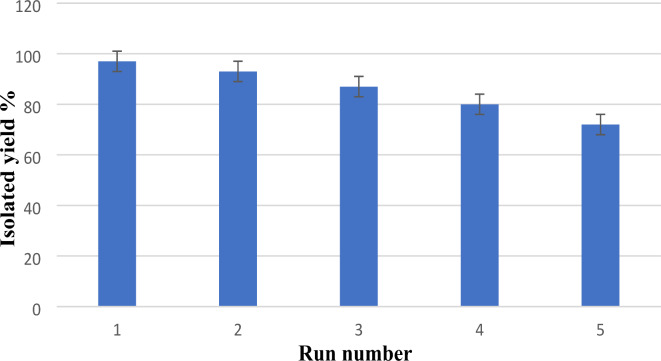
The recovering and recycling of the modified bone char were studied in the Synthesis of pyrimidine-5-carbonitrile using 4-chlorobenzaldehyde (1 mmol), malononitrile (1.2 mmol) and urea (1.8 mmol) in the presence bone char-nPrN-SO3H catalysis as model reaction. The resulting precipitate was filtered off and washed with hot ethanol (3 × 2 mL). The catalyst is recovered by simple filtration and washed several times with ethanol and water. The dried recovered catalyst was reused in the next experiment. The obtained results from recycling of modified bone char are summarized in Fig. 13. As shown this catalyst can be recycled up to 5 times without significantly loss in its catalytic activity. Also, Error bars indicate the range of data based on repeat experiments.
Fig. 13.
Recyclability of modified bone char as Bronsted acid catalyst in synthesis of pyrimidine-5-carbonitrile.
Comparison results of modified bone char with other catalysts for synthesis of 6-amino-5-cyano-4-(4chloro)-phenyl-2-mercapto pyrimidine
To extend the scope of merit of synthesized catalyst, it was compared with other catalysts for synthesis of 6-Amino-5-Cyano-4-(4Chloro)-Phenyl-2-Mercapto Pyrimidine (Table 4). The bone char catalyst shows very good reaction times and high yields. However, all reported methods have several disadvantages, including long reaction times, hard reaction conditions, and unfavorable results. Therefore, in this study, we used the cheapest and readily available natural based catalyst with the simple reaction method to synthesize pyrimidine-5-carbonitril.
Table 4.
Comparison results of modified bone char with other catalysts for synthesis of 6-Amino-5-Cyano-4-(4Chloro)-Phenyl-2-Mercapto pyrimidine.
| Entry | Condition | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|
| 1 | NH4Cl (ammonium chloride) (0.12 g), 110 °C, solvent free | 4 | 81 | 44 |
| 2 | Con. hydrochloric acid (0.3 ml), EtOH, Reflux | 4 | 51 | 45 |
| 3 | Sodium acetate (0.082 g), water, reflux | 3.5 | 76 | 46 |
| 4 | Phosphorus Pentoxide (0.05 g), EtOH, Reflux | 1 | 92 | 47 |
| 5 | CdFe2(C4H4O6)3.5H2O (0.02 g), EtOH, Reflux | 45 min | 92 | 48 |
| 6 | Modified bone char (sulfonated bonchar) (0.05 g), 80 °C, solvent free | 20 min | 95 | This work |
Conclusion
In this work, bone char was prepared from pyrolysis of bone cattle at 450 °C and applied as a catalyst support. Then functionalization by 3-aminopropyl-trietoxsysilan and sulfonation with chlorosulfonic acid made it an efficient and reusable heterogeneous solid acid biocatalyst for the one pot synthesis of pyrimidine-5-carbonitrile derivatives. Therefore, the first use of bone char as a catalyst support in multi compound organic reaction is the main novelty of this research. The presented biocatalyst was characterized by some techniques such as FT-IR, XRD, SEM, EDS, TGA, TEM and BET techniques.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the research facilities of Bu-Ali Sina University, Hamedan, Iran.
Abbreviations
- BC
Bone char
- SEM
Scanning electron microscopy
- TEM
Transmission Electron Microscopy
- EDS
Energy-Dispersive X-ray spectroscopy
- XRD
X-ray diffraction
- TGA
Thermogravimetric analysis
- FT-IR
Fourier transform infrared spectroscopy
Author contributions
Maryam Hajjami: Funding acquisition, Supervision, Project administration, Conceptualization, Resources.Zahra Siahpour: Methodology, Investigation, Writing - original draft.
Funding
The authors thank Bu-Ali Sina University for the financial support for this research.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information file].
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wan, Y. & Zhao On the controllable soft-templating approach to mesoporous silicates. Chem. Rev.10710.1021/cr068020s (2007). [DOI] [PubMed]
- 2.Tüysüz, H. & Schüth, F. Chapter two - ordered mesoporous materials as catalysts. Adv. Catal.5510.1016/B978-0-12-385516-9.00002-8 (2012).
- 3.Davis, M. E. Ordered porous materials for emerging applications. Nature41710.1038/nature00785 (2002). [DOI] [PubMed]
- 4.González-García P. activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev.8210.1016/j.rser.2017.04.117 (2018).
- 5.Mansour, F., Al-hindi, M., Yahfoufi, R., Ayoub, G. M. & Ahmad, M. N. The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: A review. Rev. Environ. Sci. Biotechnol.1710.1007/s11157-017-9456-8 (2017).
- 6.Danish, M. & Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev.8710.1016/j.rser.2018.02.003 (2018).
- 7.Tan, X. et al. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour Technol.22710.1016/j.biortech.2016.12.083 (2017). [DOI] [PubMed]
- 8.Khan, M. A., Hameed, B. H., Lawler, J., Jeon, B. H. & Kumar. M. Developments in activated functionalized carbons and their applications in water decontamination: A review, Desalin. Water Treat.5410.1080/19443994.2014.885397 (2015).
- 9.Lehmann, J. & Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation Ed (Routledge, 2015).
- 10.Zwetsloot, M. J. et al. Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae-biochar interactions. Plant. Soil.40810.1007/s11104-016-2905-2 (2016).
- 11.Dimovic, S., Smiciklas, I., Plecaš, I., Antonovic, D. & Mitric, M. Comparative study of differently treated animal bones for CO2 removal. J. Hazard. Mater.164, 18799265. 10.1016/j.jhazmat.2008.08.013 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Sternitzke, V. et al. Uptake of fluoride from aqueous solution on nano-sized hydroxyapatite: Examination of a fluoridated surface layer. Environ. Sci. Technol.4610.1021/es202750t (2012). [DOI] [PubMed]
- 13.Rojas-Mayorga, C. K. et al. Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water. J. Anal. Appl. Pyrol. 104. 10.1016/j.jaap.2013.09.018 (2013).
- 14.Cazetta, A. L. et al. And Almeida,V.C. Thermally activated carbon from bovine bone: optimization of synthesis conditions by response surface methodology. J. Anal. Appl. Pyrol. 110. 10.1016/j.jaap.2014.10.022 (2014).
- 15.Chen, Y. N., Chai, L. Y. & Shu, Y. D. Study of arsenic(V) adsorption on bone char from aqueous solution. J. Hazard. Mater.16010.1016/j.jhazmat.2008.02.120 (2008). [DOI] [PubMed]
- 16.Patel, S., Han, J., Qiu, W. & Gao, W. Synthesis and characterisation of mesoporous bone char obtained by pyrolysis of animal bones, for environmental application. J. Environ. Chem. Eng.310.1016/j.jece.2015.07.031 (2015).
- 17.Nunes-Pereira, J. et al. Highly efficient removal of fluoride from aqueous media through polymer composite membranes. Sep. Purif. Technol.20510.1016/j.seppur.2018.05.015 (2018).
- 18.Chou, C. C. & Hanson, K. R. Characteristics of bone clear Carbon. Ind. Eng. Chem. Prod. Res. Develop. 1010.1021/i360037a002 (1971).
- 19.Leyva-Ramosa, R., Rivera-Utrilla, J., Medellin-Castilloa, N. A. & Sanchez-Polob, M. Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. J. Chem. Eng.15810.1016/j.cej.2010.01.019 (2010).
- 20.Pan, X., Wang, J. & Zhang, D. Sorption of cobalt to bone char: kinetics, competitive sorption and mechanism. Desalination24910.1016/j.desal.2009.01.027 (2009).
- 21.Brown, R. C. D. Recent developments in solid phase organic synthesis. J. Chem. Soc. Perkin Trans.110.1039/A805801F (1998).
- 22.Pershin, N. G., Sherbakova, L. I., Zykova, T. N. & Sakolova, V. N. Antibacterial activity of pyrimidine and pyrrolo-(3,2-d)-pyrimidine derivatives. Farmakol.Toksikol. 35, Russian. PMID: 4626161. (1972). [PubMed]
- 23.Metolcsy, G. Structure-activity correlations and mode of action of some selected types of antifungal compounds World. Rev. Pest Contr. 10, 50 (1971). [Google Scholar]
- 24.Regnier, G. L. et al. Triphenyl propyl piper zine derivatives as new potent analgesic substances. J. Med. Chem.1510.1021/jm00273a600 (1972).
- 25.Winter, C. A., Fisley, E. A. & Nuss, G. W. Carrageenininduced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med, 111 (1962). 10.3181/00379727-111-27849 [DOI] [PubMed]
- 26.Padmaja, A., Payani, T., Reddy, G. D. & Padmavathi, V. Synthesis, antimicrobial and antioxidant activities of substituted pyrazoles, isoxazoles, pyrimidine and thioxopyrimidine derivatives. Eur. J. Med. Chem.4410.1016/j.ejmech.2009.06.024 (2009). [DOI] [PubMed]
- 27.Selvam, T. P., James, C. R., Dniandev, P. V. & Valzita, S. K. A mini review of pyrimidine and fused pyrimidine marketed drugs res. Pharm2, 2231–539X (2012). www.researchgate.net/publication/348407289 [Google Scholar]
- 28.Katritzky, A. R., Soloducho, J. & Belyakov, S. Bis-4-halophenyl-pyrimidines and – 1,2,4,5-tetrazines. Arkivoc1 (2000). ISSN 1424–6376.
- 29.Spivey, A. C., Srikaran, R., Diaper, C. M. & Turner, D. J. Traceless solid phase synthesis of 2-substituted pyrimidines using an ‘off-theshelf’ chlorogermane-functionalised resin. Org. Biomol. Chem.110.1039/B303064D (2003). [DOI] [PubMed]
- 30.Bratusek, U., Meden, A., Svete, J. & Stanovnik, B. Transformations of (Z)-2-benzoylamino-4-dimethylamino-2-oxo-3-butene and (E)- 3-benzoylamino-4-cyano-2-oxo-3-butene into pyrimidine, pyrazole and isoxazole derivatives. Arkivoc4, 1551–7012 (2003). [Google Scholar]
- 31.Moradi, P. & Hajjami, M. Magnetization of biochar nanoparticles as a novel support for fabrication of organo nickel as a selective, reusable and magnetic nanocatalyst in organic reactions. New. J. Chem.4510.1039/D0NJ04990E (2021). [DOI] [PMC free article] [PubMed]
- 32.Moradi, P. & Hajjami, M. Stabilization of ruthenium on biochar-nickel magnetic nanoparticles as a heterogeneous, practical, selective, and reusable nanocatalyst for the Suzuki C–C coupling reaction in water. RSC Adv.1210.1039/D1RA09350A (2022). [DOI] [PMC free article] [PubMed]
- 33.Moradi, P., Hajjami, M. & Tahmasbi, B. Fabricated copper catalyst on biochar nanoparticles for the synthesis of tetrazoles as antimicrobial agents. Polyhedron17510.1016/j.poly.2019.114169 (2020).
- 34.Moradi, P., Hajjami, M. & Valizadeh-Kakhki, F. Biochar as heterogeneous support for immobilization of Pd as efficient and reusable biocatalyst in C–C coupling reactions, Appl. Organomet. Chem. 33, 10.1002/aoc.5205
- 35.Gharehkhani, A. & Hajjami, M. Magnetic curcumin-copper graphene oxide as facile and recyclable heterogeneous nanocatalyst for preparation of polyhydroquinolines and sulfoxides. Sci. Rep.1410.1038/s41598-024-67876-z (2024). [DOI] [PMC free article] [PubMed]
- 36.Mansour, S. F. et al. Effect of preparation conditions on the nanostructure of hydroxyapatite and brushite phases. Appl. Nanosci.610.1007/s13204-015-0509-4 (2016).
- 37.Siddharthan, A., Seshadri, S. K. & Kumar, T. S. S. Influence of microwave power on nanosized hydroxyapatite particles, Scr. Mater.55, (2006). 10.1016/j.scriptamat.2006.03.044. (2019).
- 38.Kashif Shahid, M., Kim, J. Y., Choi, Y. G. & Groundw Synthesis of bone char from cattle bones and its application for fluoride removal from the contaminated water. Sustain. Dev.810.1016/j.gsd.2018.12.003 (2019).
- 39.Wang, P. E. & Chaki, T. K. Sintering behavior and mechanical properties of hydroxyapatite and dicalcium phosphate. J. Mater. Sci: Mater. Med.410.1007/BF00120384 (1993).
- 40.Patela, S., Hanb, J., Qiua, W. & Gaoa, W. Synthesis and characterisation of mesoporous bone char obtained by pyrolysis of animal bones, for environmental application. J. Environ. Chem. Eng.310.1016/j.jece.2015.07.031 (2015).
- 41.Carrales-Alvarado, D. H. et al. Bone char modification by iron to improve its capacity for adsorbing fluoride from an aqueous solution. Sustain. Environ. Res.3310.1186/s42834-023-00164-z (2023).
- 42.Ghrab, S., Benzina, M. & D Lambert, S. Copper adsorption from wastewater using bone charcoal. AMPC710.4236/ampc.2017.75012 (2017).
- 43.Hassani, Z. Synthesis of pyrimidine derivatives from three-component reaction of malononitrile, aldehydes and thiourea/urea in the presence of high surface area and nanosized MgO as a highly effective heterogeneous base catalyst. Lett. Org. Chem.1110.2174/1570178611666140313005406 (2014).
- 44.Aher, J. S. et al. One pot synthesis of pyrimidine-5-carbonitrile and pyrimidine-5-carboxamide using ammonium chloride under solvent free condition. J. Chem. Sci.13110.1007/s12039-019-1633-6 (2019).
- 45.Bhuva, N. H. et al. Synthesis of novel 2-mercapto-4-(p-aminophenyl sulphonylamino)-6-(aryl)-pyrimidine-5-carboxamide derivatives via the biginelli reaction. ILCPA3610.18052/www.scipress.com/ILCPA.36.168 (2014).
- 46.Sureja1, D. K., Dholakia, S. P. & Vadalia, K. R. Aqua mediated sodium acetate catalysed one-pot synthesis of pyrimidine derivatives as anti-inflammatory and antioxidant agent, Der Pharma Chemica8(9), (2016). http://derpharmachemica.com/archive.html) ISSN 0975-413X.
- 47.Patil, D. R., Salunkhe, S. M., Deshmukh, M. B. & Anbhule, P. V. One step synthesis of 6-amino-5-cyano-4-phenyl-2-mercapto pyrimidine using phosphorus pentoxide. Open. Catal. J.310.2174/1876214X01003010083 (2010).
- 48.Mahesh, W. The Green synthesis of 6-amino-5-cyano-4-phenyl-2-hydroxy and mercapto pyrimidine derivatives. IJARSCT310.48175/IJARSCT-8126 (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information file].