FIGURE 1.
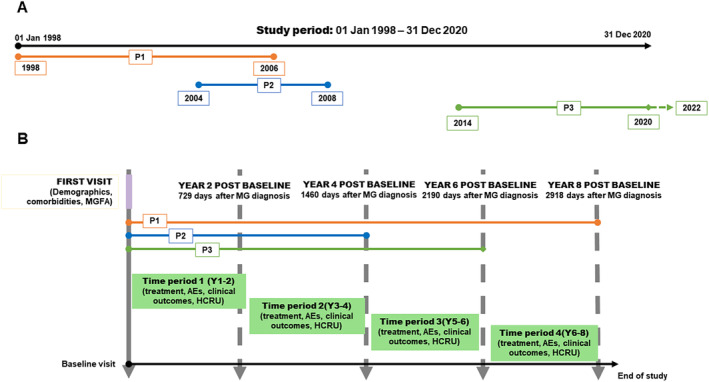
Study design and example. Upper panel (A) shows an example of three hypothetical patients (P1, P2 and P3) included in our study. P1 had a complete 8‐year follow‐up. P2 had an incomplete 4‐year follow‐up because of attrition or death. P3 had an incomplete 6‐year follow‐up because of the end of study period. Lower panel (B) illustrates the study design from the baseline visit, with biannual periods (time periods) of observation. It also represents the aforementioned hypothetical patients organized from first visit to our center, considered as the baseline study visit. From the first visit or baseline visit data on clinical outcomes, treatments, adverse effects and healthcare resource utilization were collected for every biannual period. Data were collected biennially until patients reached 8 years of follow‐up, follow‐up reached 31 December 2020 or the patient's death. AE, adverse effect; HCRU, healthcare resource utilization; MG, myasthenia gravis; MGFA, Myasthenia Gravis Foundation of America; P, patient; Y, year.
