FIGURE 3.
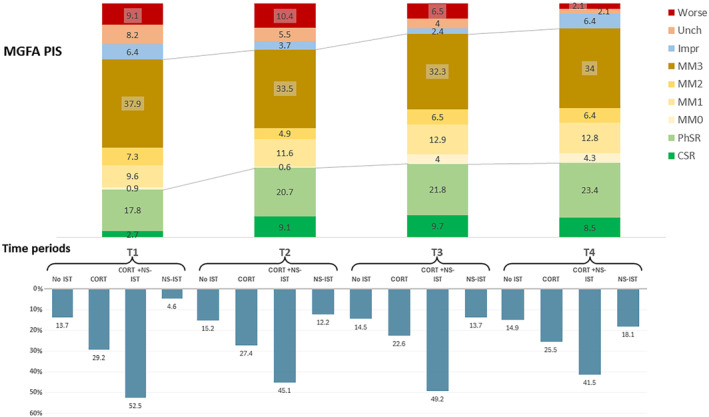
Drug use and clinical outcome (Myasthenia Gravis Foundation of America post‐interventional status, MGFA‐PIS) in each time period. The upper panel shows MGFA‐PIS at each study period. The lower panel shows information about immunosuppressant therapy use at each study time period. CORT + NS‐IST, combination of corticosteroids and nonsteroidal IST; CORT, corticosteroids; CSR, clinical stable remission; Impr, improved; MM, minimal manifestations; No IST, no immunosuppressant therapies, but may be using pyridostigmine; NS‐IST, nonsteroidal IST; PhSR, pharmacologic stable remission; Unch, unchanged.
