Abstract
Patient: Male, 60-year-old
Final Diagnosis: Herpes simplex virus 2 hepatitis
Symptoms: Generalized weakness • chills • fever
Clinical Procedure: —
Specialty: Infectious Diseases • General and Internal Medicine
Objective:
Rare disease
Background:
Herpes simplex virus (HSV) is a rare cause of hepatitis. HSV hepatitis can be life-threatening due to its rapid progression to liver failure if not treated on time. It affects primarily immunocompromised individuals but can also present in immunocompetent hosts. HSV hepatitis in solid organ transplant recipients usually occurs in the early post-transplant period as fulminant hepatitis. We present a rare case of febrile anicteric HSV2 hepatitis occurring late in the post-transplant period, with only mild elevation in transaminase levels.
Case Report:
A 60-year-old man presented to the Emergency Department with generalized weakness, chills, and fever for 1 day. His medical history included Crohn’s disease, primary sclerosing cholangitis, liver transplantation, and cholangiocarcinoma. Initial laboratory findings revealed leukocytosis. Extensive workup did not reveal a clear etiology of persistent fever. Liver enzymes peaked to aspartate transaminase 198 U/L and alanine transaminase 135 U/L, suggesting possible hepatitis. Liver biopsy showed focal areas of necrosis with vague histiocyte collections. Liver biopsy tissue was positive for HSV2 by polymerase chain reaction; therefore, HSV2 hepatitis diagnosis was made. Intravenous acyclovir was initiated for treatment of HSV2 hepatitis, which resulted with fever resolution within 48 h of initiation and return of liver enzymes to normal levels.
Conclusions:
This case highlights the importance of having a high suspicion of HSV hepatitis as a rare cause of persistent fevers in immunosuppressed, post-transplant patients even in the late post-transplant period and in the absence of mucocutaneous lesions. Prompt recognition of this disease is crucial to start prompt treatment and decrease mortality.
Key words: Hepatitis; Herpesvirus 2, Human; Immunocompromised Host; Transplant Recipients
Introduction
Herpes simplex virus 2 (HSV2) is a sexually transmitted disease with an annual incidence of 1 million cases per year [1]. Only 1% of all cases of acute liver failure are caused by HSV hepatitis [2]. HSV hepatitis mainly occurs in immunocompromised patients, including solid organ transplant (SOT) recipients, patients receiving immunosuppressive medications or chemotherapy, patients with immunodeficiency syndrome or HIV infection, pregnant patients, patients after thymectomy, or even immunocompetent hosts [3–7]. Post-transplant HSV hepatitis usually occurs in the early post-transplant period as reactivation of latent infection or primary donor-derived infection and, only in rare instances, occurs de novo [8–10]. Most often, the reported signs and symptoms of HSV hepatitis are fever, highly abnormal liver function tests without jaundice, right superior quadrant abdominal pain, and leukopenia [8]. In SOT recipients, mucocutaneous lesions make up to 85% of HSV infections, but other organ involvement can be seen, causing esophagitis, hepatitis, encephalitis, hepatitis, or disseminated infection [9,10]. Clinical presentation of HSV hepatitis closely resembles that of other etiologies of acute hepatitis, posing a challenge for clinicians to consider HSV as the underlying diagnosis of persistent fever and to initiate timely treatment, particularly when liver function tests are not markedly abnormal, or the patient has no presence of characteristic HSV mucocutaneous lesions. In addition, HSV hepatitis rarely occurs in the late post-SOT period in the absence of increased immunosuppressive therapy. Treatment of HSV infection includes acyclovir or alternative therapy with valacyclovir and ganciclovir, or foscarnet and cidofovir in the case of resistance [9,11]. HSV2 hepatitis has particularly high mortality if not treated on time [12]. Therefore, it is important to increase awareness of this rare etiology of hepatitis to avoid its characteristic rapid progression to liver failure and to decrease mortality. Here, we present a case of a patient 2 years after liver transplant who was found to have HSV2 hepatitis as a cause of persistent fevers.
Case Report
A 60-year-old man patient presented to the Emergency Department at Mayo Clinic Florida with generalized weakness, chills, and fevers up to 39.7°C for 1 day. His past medical history consisted of Crohn’s disease, primary sclerosing cholangitis, liver transplantation 2 years ago, cholangiocarcinoma, and Whipple procedure with intraoperative radiation. He received the last neoadjuvant chemotherapy 2 months before and last radiation therapy 2 weeks before this admission. His chronic antirejection regimen consisted of tacrolimus 2 mg twice a day. The patient had negative HSV1 and HSV2 blood polymerase chain reaction (PCR) tests prior to his liver transplant and had completed 6 months of antiviral prophylaxis after the liver transplant.
His vital signs on admission were temperature of 39.4°C, heart rate of 92 beats per min, respiratory rate of 18 breaths per min, blood pressure of 109/75 mmHg, and oxygen saturation of 100% on room air. His physical examination was remarkable for a benign abdomen, with no abdominal tenderness and no palpable hepatosplenomegaly. He had no oral or genital mucocutaneous lesions. Initial workup of laboratory findings and imaging was remarkable for stable chronic anemia, leukocytosis with neutrophilia, mild alanine transaminase (ALT)-predominant transaminitis, mild prolonged prothrombin time/elevated international normalized ratio, and elevated ferritin. All results are summarized in Table 1. Blood and urine cultures and hepatitis A, B, and C serologies were ordered. The patient was admitted for evaluation of fever of an unclear source in an immunocompromised host. Empiric piperacillin-tazobactam was initiated.
Table 1.
Summary of laboratory and imaging workup.
| Laboratory workup (normal values) | Time of admission | Day 4* | Day 5* | Day 6* | Day 12* | Day 14: 2 days after antiviral treatment | 4 weeks after hospital discharge |
|---|---|---|---|---|---|---|---|
| Leukocytes (3.4–9.6×109/L) | 11.7 | 1.6 | 2.3 | 1.9 | 2.2 | 2.9 | 4.1 |
| Absolute neutrophil count (1.56–6.45×109/L) | 9.50 | 1.53 | 1.03 | 1.12 | 1.52 | 2.30 | 2.63 |
| Hemoglobin (13.2–16.6 g/dL) | 9.8 | 7.5 | 8.6 | 7.9 | 7.7 | 7.2 | 9.5 |
| Platelet count (135–317×109/L) | 187 | 98 | 100 | 95 | 106 | 123 | 129 |
| AST (8–48 U/L) | 48 | 174 | 175 | 198 | 118 | 60 | 48 |
| ALT (7–55 U/L) | 57 | 126 | 138 | 135 | 143 | 112 | 49 |
| Total bilirubin (<1.2 mg/dL) | 0.6 | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 |
| PT (9.4–12.5 s)/INR (0.9–1.1) | 17.3/1.6 | – | 27.3/2.5 | – | 18.9/1.7 | 23.3/2.1 | 14.7/1.1 |
| Ferritin (24–336 mcg/L) | 2507 | – | 5425 | – | – | – | – |
| Fever workup on admission | Peripheral blood cultures: No growth. Urine culture: No growth. MRSA PCR swab: negative. Hepatitis A antibodies: negative. Hepatitis B serology: nonimmune. Hepatitis C antibody screen: negative. Epstein-Barr Virus PCR <2000 copies/mL | ||||||
| Imaging workup on admission | Chest X-ray: no acute abnormalities. CT of abdomen and pelvis with IV contrast: Interval pancreaticoduodenectomy and retroperitoneal lymphadenopathy with postoperative changes, without changes related to an acute intraabdominal infection. Ultrasound abdomen limited with abdominal Doppler: small thrombus within a superior mesenteric venous branch | ||||||
| Additional fever workup | Stool enteric pathogen culture stool: negative. ESR 16 (0–22 mm/1 h). Creatine kinase 41 (39–308 U/L). LDH 309 (122–222 U/L). QuantiFERON TB gold negative. HIV Ag/Ab screen: negative. Antinuclear Ab 0.9 (≤1.0 U). Rheumatoid factor 14 (<15 IU/mL). Serum electrophoresis: no apparent monoclonal protein. Serum free light chains: negative. CMV DNA PCR undetected. Adenovirus plasma PCR negative. HTLV I/II DNA not detected. Peripheral blood flow cytometry: no abnormal cells. Bone marrow biopsy: Polytypic B lymphocytes and no increase in blasts. Cryptococcal serum Ag: negative. Toxoplasma serum Ab: negative. Parvovirus B19 serum Ab: IgG positive, IgM negative. Parvovirus B19 PCR negative. Varicella-Zoster Ab negative. West and East Equine encephalitis serum Ab negative. St Louis Encephalitis serum Ab negative. Bartonella Ab panel negative. HSV1 blood PCR negative. Liver biopsy: please see Figure 1. HSV2 blood PCR positive | ||||||
| Additional imaging workup | CT chest without IV contrast: No pulmonary mass or consolidation. Transthoracic echocardiogram: No valvular heart disease or pericardial effusion. No intracardiac mass or thrombus. Venous ultrasound lower and upper extremities: negative for deep venous thrombosis. MRI abdomen without and with IV contrast: No evidence of hepatic abscess. Inflammatory appearing enhancement of the central liver favored to reflect posttreatment change. Brain MRI without and with IV contrast: Chronic small vessel disease and no other acute change. PET CT whole body FDG: Generalized moderate uptake throughout porta hepatis and adjacent hepatic parenchyma which is likely reactive to surgery. A few nonenlarged FDG avid mediastinal, right supraclavicular and left cervical lymph nodes which are likely reactive as well | ||||||
Days 4 to 12 are prior to antiviral treatment. Antiviral treatment was started on day 14. Ab – antibody; ALT – alanine transaminase; AST – aspartate transaminase; HSV – herpes simplex virus; CMV – cytomegalovirus; ESR – erythrocyte sedimentation rate; INR – international normalized ratio; LDH – lactate dehydrogenase; MRSA – methicillin-resistant Staphylococcus aureus; PT – prothrombin time; PCR – polymerase chain reaction; MRI – magnetic resonance imaging; PET CT – positron emission tomography-computed tomography; FDG – fluorodeoxyglucos.
The patient developed pancytopenia, worsening transaminitis, with aspartate transaminase (AST) peaking up to 198 U/L and ALT up to 135 U/L, without cholestatic pattern, and the rest of the workup remained unrevealing. A Doppler ultrasound of the abdomen revealed a small thrombus within a superior mesenteric venous branch, for which the patient was started on anticoagulation. The initial imaging and laboratory workup was completed, as described in Table 1. Antibiotics were discontinued, given the negative infectious workup for bacterial infection. The patient then developed acute encephalopathy on day 5 of his hospital stay, with confusion, lethargy, and non-bloody diarrhea. Diarrhea was transient and non-infectious and likely related to previous antibiotic administration. Acute encephalopathy was metabolic, due to dehydration and resolved after fluid resuscitation. Extensive additional fever workup was completed, as described in Table 1. Magnetic resonance imaging of the abdomen showed nonspecific inflammatory changes in the central liver area. Positron emission tomography-computed tomography scan also showed increased nonspecific uptake throughout the porta hepatis and adjacent hepatic parenchyma, possible reactive to prior surgery, and reactive lymphadenopathy. Given no clear source of fevers, the unrevealing workup, acute encephalopathy, and persistent transaminitis, a liver biopsy was performed, showing focal areas of necrosis with vague histiocyte collections, was negative for rejection, and was characteristic for inflammation and necrosis (Figures 1, 2). Further results revealed liver biopsy tissue PCR was positive for HSV2. HSV1 blood PCR was negative, but serum HSV2 blood PCR was positive. The patient received a diagnosis of acute HSV2 hepatitis. Intravenous acyclovir at 10 mg/kg every 8 h was initiated. The patient became afebrile 24 h later, followed by resolution of encephalopathy over the next 48 h. There was also gradual improvement of transaminitis and pancytopenia, as shown in Table 1. The patient was discharged from the hospital on day 14 of his hospital stay, with a plan to complete 21 days of valacyclovir 1 g per oral 3 times a day.
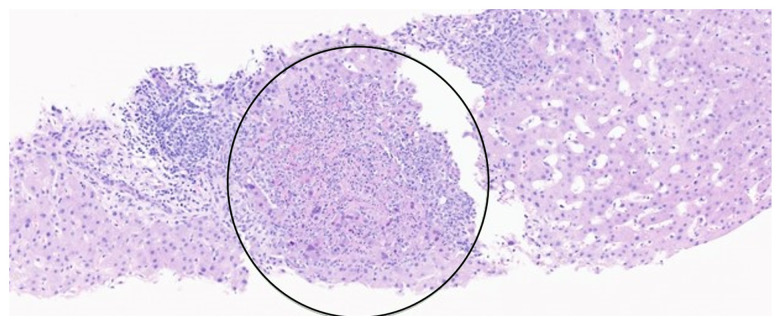
Figure 1.
Liver biopsy. Hematoxylin and eosin staining ×500. Black arrows point to HSV viral cytopathic effect around the focus of lobular necrosis, characterized by multinucleation and margination of chromatin.
Figure 2.
Liver biopsy. Hematoxylin and eosin staining ×500. Black circled area marks an area of lobular necrosis.
Discussion
HSV1 infection usually manifests as herpes labialis, and HSV2, as urogenital lesions. However, they can both cause infection in either location. HSV primary infection presents in 30% to 50% of the patients with painful mucocutaneous vesicular lesions [12]. In our case, the patient did not have mucocutaneous lesions. When left untreated, HSV can cause different types of complications, such as aseptic meningitis and transverse myelitis, or rarely, hepatitis, which is more commonly seen in immunosuppressed patients and pregnant patients in their third trimester [3]. Hepatitis related to HSV has a characteristic of rapid progression, leading to acute liver failure in 74% of patients [4] and high mortality of up to 90% [12]. Unfortunately, orthotopic liver transplantation of patients with HSV-induced liver failure has a high risk of failure and death because of generalized infection [4]. HSV hepatitis represents only 1% to 2% of all acute liver failure cases [2,13]. Given the low clinical suspicion for the disease, most cases are diagnosed postmortem [2], and diagnosed in only 26% of non-pregnant patients antemortem [5]. Mortality significantly decreases if treatment is initiated with intravenous acyclovir within 4.2 days of symptoms onset [2]. Therefore, clinicians need to consider starting empirical treatment in some patients with acute liver failure, risk factors for HSV infection, and no clear etiology of the symptoms even before test results could be obtained.
Initial symptoms are nonspecific and can include fever, chills, myalgia, and abdominal pain. Encephalopathy is considered a late manifestation. The most common laboratory findings are transaminitis (100–1000-fold above normal), with low to normal total bilirubin, known as “anicteric transaminitis”, leukopenia, thrombocytopenia, coagulopathy, and disseminated intravascular coagulation [3]. Liver imaging is nonspecific and can show different signs of liver enlargement and periportal edema [14]. HSV serology is not particularly useful for prompt diagnosis, given the high rate of false-negative and false-positive test results. HSV PCR is a better test than HSV serology in patients with acute liver failure of an unclear source. In our case, this test was not considered until liver biopsy results revealed HSV2 infection. Liver biopsy is the criterion standard for diagnosis of HSV hepatitis. Pathological findings are characterized by areas of inflammation, hemorrhagic piecemeal necrosis, and enlarged ground glass nuclei hepatocytes with marginalized chromatin [15], as seen in our case. HSV hepatitis has 2 pathological forms, focal and diffuse, with focal HSV hepatitis associated with a better outcome [16].
Transplant patients are at significantly elevated risk of de novo viral infections, due to a long-term immunosuppressed state, compared with immunocompetent individuals [8]. HSV infections in initially HSV-negative SOT recipients can be donor-derived infections or community-acquired primary infection. Donor-derived HSV infection occurs early in the post-SOT period, coinciding with a period of maximum immunosuppression by induction therapy [10,17]. There have been only 2 reported cases in which HSV1 hepatitis occurred in the late post-transplant period, observed after 4 and 5 years after transplantation, and both were associated with an increase in immunosuppression (corticosteroids or thymoglobulin) as therapy against graft rejection [10]. It is suspected that our patient had a rare case of de novo HSV2 viral infection, since he had negative HSV tests at the time of liver transplant, he completed required post-transplant antiviral prophylaxis, HSV hepatitis occurred in late post-transplant period, and he did not have a recent increase in immunosuppressive therapy. The exposure and source of HSV2 infection remain unknown, due to lack of exposure in history and lack of mucocutaneous lesions.
The variables on presentation associated with death or need for liver transplant are male sex, age >40 years, immunocompromised state, ALT >5000 U/L, platelet count <75×103/L, coagulopathy, encephalopathy, and absence of viral therapy [2]. Even though the present patient had some of the poor prognostic factors (male, immunocompromised, older than 40 years) and was without immediate acyclovir administration (13 days from fever onset), initiation of acyclovir led to a favorable outcome, with resolution of fever, encephalopathy, and hepatitis, as well as survival now 2 years at this moment, after presentation for HSV hepatitis.
HSV hepatitis after liver transplantation is more frequently seen within 20±12 days after transplantation [8], suggesting reactivation of the virus in the recipient or a primary infection acquired from the donor. A single-institution retrospective study of HSV hepatitis after SOT published in 1991 evaluated data from 1980 to 1988, in the period before HSV prophylaxis was part of standard post-transplant care. The study reported 12 cases of HSV hepatitis that occurred a median of 18 days (range 5–46 days) after transplant, out of which, only 1 case, with disseminated HSV disease, occurred after 2 years after heart transplantation [16]. In a systematic review of HSV hepatitis cases from 1969 to 2006, which included 131 cases (after 6 cases were excluded), 30% were immunocompromised from a prior solid organ or hematopoietic cell transplant, and these patients had an average time of disease onset after transplantation of 205 days (±526) [2]. Late onset of HSV hepatitis 2 years after SOT is rare, and in our patient, might be explained by a further immunosuppressed state after receiving chemotherapy 2 months prior to the disease onset.
Treatment of HSV hepatitis consists of high-dose intravenous acyclovir 5 to 10 mg/kg every 8 h for at least 48 h, until clinical improvement is noted, followed by oral antiviral therapy to complete a total of 10 to 28 days of therapy. Early testing and treatment are important to increase the chances of favorable outcome and reduce mortality. Providers should consider administering acyclovir in patients with acute or impending liver failure without known etiology. Acyclovir should be administered even in patients without mucocutaneous lesions and with only mild liver enzyme elevation in early stages of HSV viremia, HSV hepatitis, or fever, especially if a patient is immunocompromised.
Conclusions
SOT recipients and immunocompetent patients with persistent fever, despite initial negative infectious workup or who are not responding to the initial treatment, should be evaluated for HSV infection even if liver function test results are 2 to 3 times the upper limit of normal. Due to a lack of presence of mucocutaneous lesions and only mild elevation of transaminases, HSV hepatitis is not often suspected early in the disease in patients with persistent unexplained fever. This can have fatal consequences, due to rapid progression to disseminated HSV infection or development of fulminant HSV hepatitis. Early diagnosis and initiation of empiric treatment for HSV hepatitis should lead to a favorable outcome.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: An analysis of the published literature and institutional cases. Liver Transpl. 2007;13(10):1428–34. doi: 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A, Granillo A, Burns E, et al. Herpes simplex virus-2 hepatitis: A case report and review of the literature. Case Rep Med. 2020;2020:8613840. doi: 10.1155/2020/8613840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riediger C, Sauer P, Matevossian E, et al. Herpes simplex virus sepsis and acute liver failure. Clin Transplant. 2009;23(Suppl. 21):37–41. doi: 10.1111/j.1399-0012.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 4.Aboguddah A, Stein HB, Phillips P, et al. Herpes simplex hepatitis in a patient with psoriatic arthritis taking prednisone and methotrexate. Report and review of the literature. J Rheumatol. 1991;18(9):1406–12. [PubMed] [Google Scholar]
- 5.Lynch DT, Bruns K, Feig JA. HSV Hepatitis in an Immunocompetent Adult. Am J Forensic Med Pathol. 2024;45(1):e8–e10. doi: 10.1097/PAF.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan D, Kaul CM, Buttar AB, et al. Disseminated herpes simplex virus-2 (HSV-2) as a cause of viral hepatitis in an immunocompetent host. Am J Case Rep. 2021;22:e932474. doi: 10.12659/AJCR.932474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Côté-Daigneault J, Carrier FM, Toledano K, et al. Herpes simplex hepatitis after liver transplantation: Case report and literature review. Transpl Infect Dis. 2014;16(1):130–34. doi: 10.1111/tid.12178. [DOI] [PubMed] [Google Scholar]
- 8.Jothimani D, Venugopal R, Vij M, Rela M. Post liver transplant recurrent and de novo viral infections. Best Pract Res Clin Gastroenterol. 2020;46–47:101689. doi: 10.1016/j.bpg.2020.101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arana C, Cofan F, Ruiz P, et al. Primary herpes simplex virus type 1 infection with acute liver failure in solid organ transplantation: Report of three cases and review. IDCases. 2022;28:e01485. doi: 10.1016/j.idcr.2022.e01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DH, Zuckerman RA, AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9)(e13526) doi: 10.1111/ctr.13526. [DOI] [PubMed] [Google Scholar]
- 11.Poley RA, Snowdon JF, Howes DW. Herpes simplex virus hepatitis in an immunocompetent adult: A fatal outcome due to liver failure. Case Rep Crit Care. 2011;2011:138341. doi: 10.1155/2011/138341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichai P, Roque Afonso AM, Sebagh M, et al. Herpes simplex virus-associated acute liver failure: A difficult diagnosis with a poor prognosis. Liver Transpl. 2005;11(12):1550–55. doi: 10.1002/lt.20545. [DOI] [PubMed] [Google Scholar]
- 13.Rofsky NM, Fleishaker H. CT and MRI of diffuse liver disease. Semin Ultrasound CT MR. 1995;16(1):16–33. doi: 10.1016/0887-2171(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 14.Natu A, Iuppa G, Packer CD. Herpes simplex virus hepatitis: A presentation of multi-institutional cases to promote early diagnosis and management of the disease. Case Reports Hepatol. 2017;2017:3180984. doi: 10.1155/2017/3180984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusne S, Schwartz M, Breinig MK, et al. Herpes simplex virus hepatitis after solid organ transplantation in adults. J Infect Dis. 1991;163(5):1001–7. doi: 10.1093/infdis/163.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhold I, Teasca L, Rodriguez ER, et al. Swiss Transplant Cohort Study (STCS). Donor-derived fulminant herpes simplex virus hepatitis after liver transplantation: Two cases and review of literature. Transpl Infect Dis. 2023;25(4):e14080. doi: 10.1111/tid.14080. [DOI] [PubMed] [Google Scholar]