Abstract
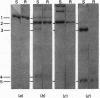
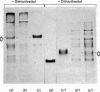
A peptidase activity capable of excising in a single fragment the N-terminal extension of the precursor of collagen type III (p-N-collagen type III) was observed in calf tendon fibroblast culture medium. A new procedure was developed for detecting this peptidase (p-N-collagen type III peptidase). It is based on the use of 14C-labelled p-N-collagen type III obtained by carboxymethylation of the half-cystine residues with iodo-[14C]acetamide. The released labelled N-terminal extension is soluble in 27% (v/v) ethanol, whereas the uncleaved substrate and the collagen are precipitated under these conditions. The endopeptidase nature of p-N-collagen type III peptidase is supported by the similarity in molecular weight of the product of cleavage of p-N-collagen III by the enzyme to those obtained by cleavage with bacterial collagenase. An apparent Km of 0.3 X 10(-6)M was established. The pH optimum of p-N-collagen type III peptidase is similar to that of p-N-collagen type I peptidase, i.e. about 7.5. Both peptidases are inhibited by dithiothreitol and by Cu2+ and Zn2+, but not by other bivalent ions. p-N-collagen type III peptidase does not cleave p-N-collagen I or p-N-gelatin I. Partial purification of p-N-collagen type III peptidase from fibroblast culture medium was performed by sieve chromatography on Ultrogel AcA-34 to yield two peaks of activity, of mol.wts. 170000 and 100000. Part of the activity was retained on affinity chromatography on concanavalin A--Sepharose. Studied as a function of the age of the culture, p-N-collagen type III peptidase activity produced by tendon fibroblasts parallels that of p-N-collagen type I peptidase and collagen synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Bächinger H. P., Timpl R., Engel J. Three conformationally distinct domains in the amino-terminal segment of type III procollagen and its rapid triple helix leads to and comes from coil transition. Eur J Biochem. 1978 Oct 16;90(3):595–603. doi: 10.1111/j.1432-1033.1978.tb12640.x. [DOI] [PubMed] [Google Scholar]
- Byers P. H., McKenney K. H., Lichtenstein J. R., Martin G. R. Preparation of type III procollagen and collagen from rat skin. Biochemistry. 1974 Dec 3;13(25):5243–5248. doi: 10.1021/bi00722a030. [DOI] [PubMed] [Google Scholar]
- Engel J., Bruckner P., Becker U., Timpl R., Rutschmann B. Physical properties of the amino-terminal precursor-specific portion of type I procollagen. Biochemistry. 1977 Sep 6;16(18):4026–4033. doi: 10.1021/bi00637a014. [DOI] [PubMed] [Google Scholar]
- Glanville R. W., Allmann H., Fietzek P. P. Cysteine residues mark the C-terminal triple-helical/non-triple-helical junction in type III collagen. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1663–1665. [PubMed] [Google Scholar]
- Hörlein D., Fietzek P. P., Kühn K. Pro-Gln: the procollagen peptidase cleavage site in the alpha1(I) chain of dermatosparactic calf skin procollagen. FEBS Lett. 1978 May 15;89(2):279–282. doi: 10.1016/0014-5793(78)80236-1. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Isersky C., Zupnik J., Lenaers A., Lee G., Lapiére C. M. Calf tendon procollagen peptidase: its purification and endopeptidase mode of action. Proc Natl Acad Sci U S A. 1974 Jan;71(1):40–44. doi: 10.1073/pnas.71.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapière C. M., Lenaers A., Kohn L. D. Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3054–3058. doi: 10.1073/pnas.68.12.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers A., Lapiere C. M. Type III procollagen and collagen in skin. Biochim Biophys Acta. 1975 Jul 21;400(1):121–131. doi: 10.1016/0005-2795(75)90132-4. [DOI] [PubMed] [Google Scholar]
- MANDL I., MACLENNAN J. D., HOWES E. L. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest. 1953 Dec;32(12):1323–1329. doi: 10.1172/JCI102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusgens B., Lapiere C. M. A simplified procedure for measuring amino-procollagen peptidase type I. Anal Biochem. 1979 Jun;95(2):406–412. doi: 10.1016/0003-2697(79)90747-4. [DOI] [PubMed] [Google Scholar]
- Shinkai H., Kawamoto T., Hori H., Nagai Y. A complex of collagenase with low molecular weight inhibitors in the culture medium of embryonic chick skin explants. J Biochem. 1977 Jan;81(1):261–263. doi: 10.1093/oxfordjournals.jbchem.a131444. [DOI] [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kivirikko K. I., Prockop D. J. Partial purification and characterization of a neutral protease which cleaves the N-terminal propeptides from procollagen. Biochemistry. 1978 Jul 25;17(15):2948–2954. doi: 10.1021/bi00608a002. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Evanson J. M. Collagenase and its natural inhibitors in relation to the rheumatoid joint. Connect Tissue Res. 1977;5(1):31–35. doi: 10.3109/03008207709152609. [DOI] [PubMed] [Google Scholar]