Abstract
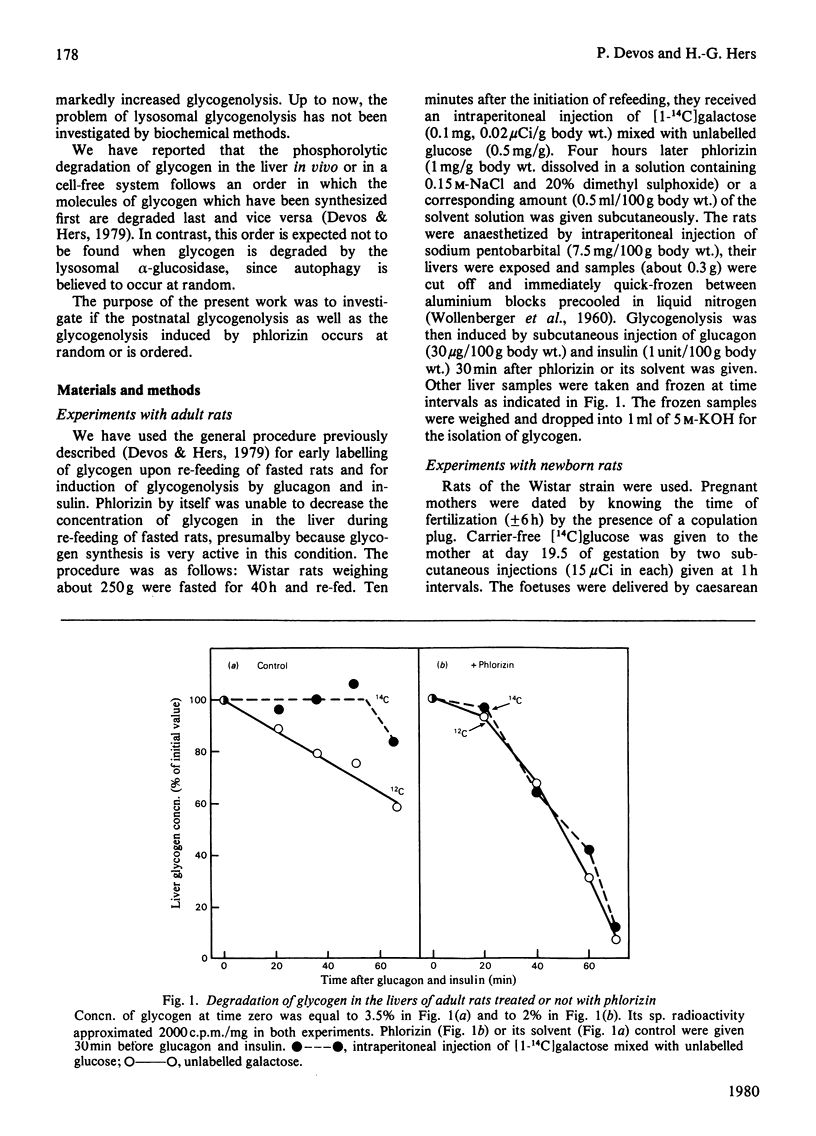
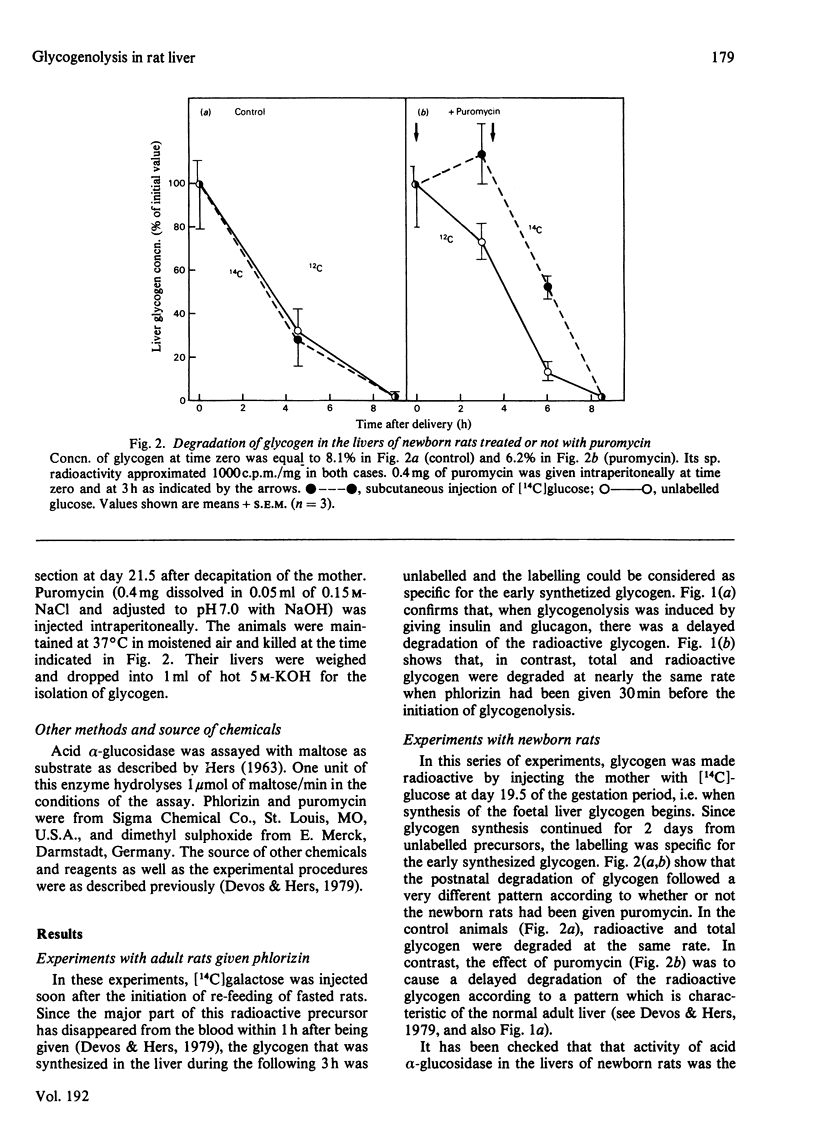
1. The glycogen formed in the livers of adult rats was labelled by injection of [1-14C] galactose soon after initiation of re-feeding after starvation. The rats were anaesthetized 4h later and glycogenolysis was induced by giving them a mixture of glucagon and insulin. In confirmation of previous work [Devos & Hers (1979) Eur J. Biochem. 99, 161-167],, there was a delay in degradation of the labelled glycogen by comparison with total glycogen. This pattern is considered as characteristic of an ordered glycogenolysis. Treatment of rats with phlorizin abolished the difference between the fate of labelled and total glycogen, causing, therefore, a random glycogenolysis. 2. Foetal liver glycogen was made radioactive by injecting [14C] glucose into the mother at the 19.5 day of gestation, i.e. at the time when this glycogen starts to be synthesized. During the postnatal degradation of this glycogen, radioactive and total glycogen were degraded at approximately the same rate, indicating that glycogenolysis occurred at random. In contrast, when puromycin was injected into the newborn rats, there was a delay in he degradation of the labelled glycogen as compared with that of total glycogen, as currently observed in the normal adult liver. 3. These data are discussed in relation with the fact that glycogen-filled vacuoles are currently seen in the livers of adult rats treated with phlorizin, and also in the neonatal livers, and that puromycin is known to cause the disappearance of these autophagic pictures in the liver of newborn rats. It is suggested that random glycogenolysis occurs through hydrolysis by the lysosomal acid alpha-glucosidase, in the course of autophagy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUDHUIN P., HERS H. G., LOEB H. AN ELECTRON MICROSCOPIC AND BIOCHEMICAL STUDY OF TYPE II GLYCOGENOSIS. Lab Invest. 1964 Sep;13:1139–1152. [PubMed] [Google Scholar]
- Becker F. F., Cornwall C. C., Jr Phlorizin induced autophagocytosis during hepatocytic glycogenolysis. Exp Mol Pathol. 1971 Feb;14(1):103–109. doi: 10.1016/0014-4800(71)90056-6. [DOI] [PubMed] [Google Scholar]
- DROCHMANS P. [Morphology of glycogen. Electron microscopic study of the negative stains of particulate glycogen]. J Ultrastruct Res. 1962 Apr;6:141–163. doi: 10.1016/s0022-5320(62)90050-3. [DOI] [PubMed] [Google Scholar]
- Deter R. L., De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967 May;33(2):437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos P., Hers H. G. A molecular order in the synthesis and degradation of glycogen in the liver. Eur J Biochem. 1979 Aug 15;99(1):161–167. doi: 10.1111/j.1432-1033.1979.tb13242.x. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofert J. F., Boutwell R. K. Effect of puromycin on hepatic glycogen phosphorylase activity. Proc Soc Exp Biol Med. 1966 Feb;121(2):532–536. doi: 10.3181/00379727-121-30822. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. I. Purification and properties of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1403–1415. doi: 10.1021/bi00808a015. [DOI] [PubMed] [Google Scholar]
- Jézéquel A. M., Arakawa K., Steiner J. W. The fine structure of the normal, neonatal mouse liver. Lab Invest. 1965 Nov;14(11):1894–1930. [PubMed] [Google Scholar]
- Kotoulas O. B., Phillips M. J. Fine structural aspects of the mobilization of hepatic glycogen. I. Acceleration of glycogen breakdown. Am J Pathol. 1971 Apr;63(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- LEJEUNE N., THINES-SEMPOUX D., HERS H. G. Tissue fractionation studies. 16. Intracellular distribution and properties of alpha-glucosidases in rat liver. Biochem J. 1963 Jan;86:16–21. doi: 10.1042/bj0860016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. J., Unakar N. J., Doornewaard G., Steiner J. W. Glycogen depletion in the newborn rat liver: an electron microscopic and electron histochemical study. J Ultrastruct Res. 1967 Apr;18(1):142–165. doi: 10.1016/s0022-5320(67)80236-3. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]


