Abstract
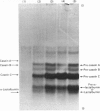
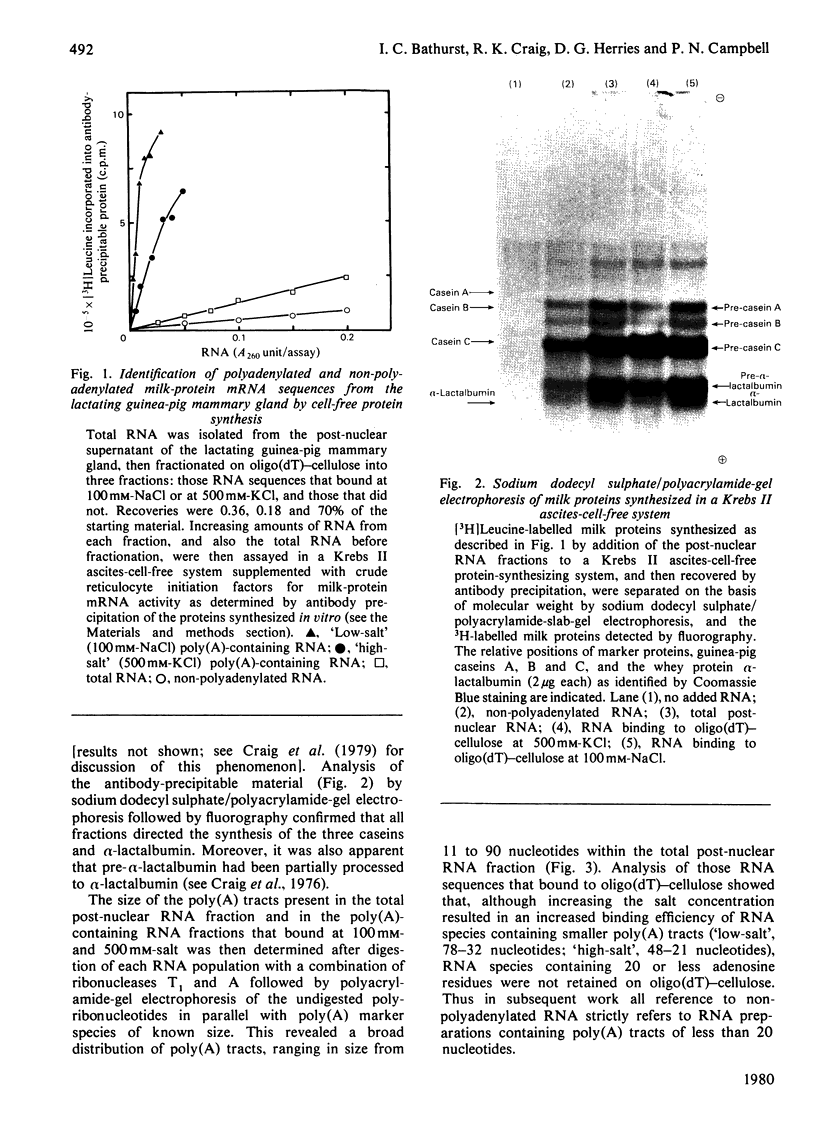
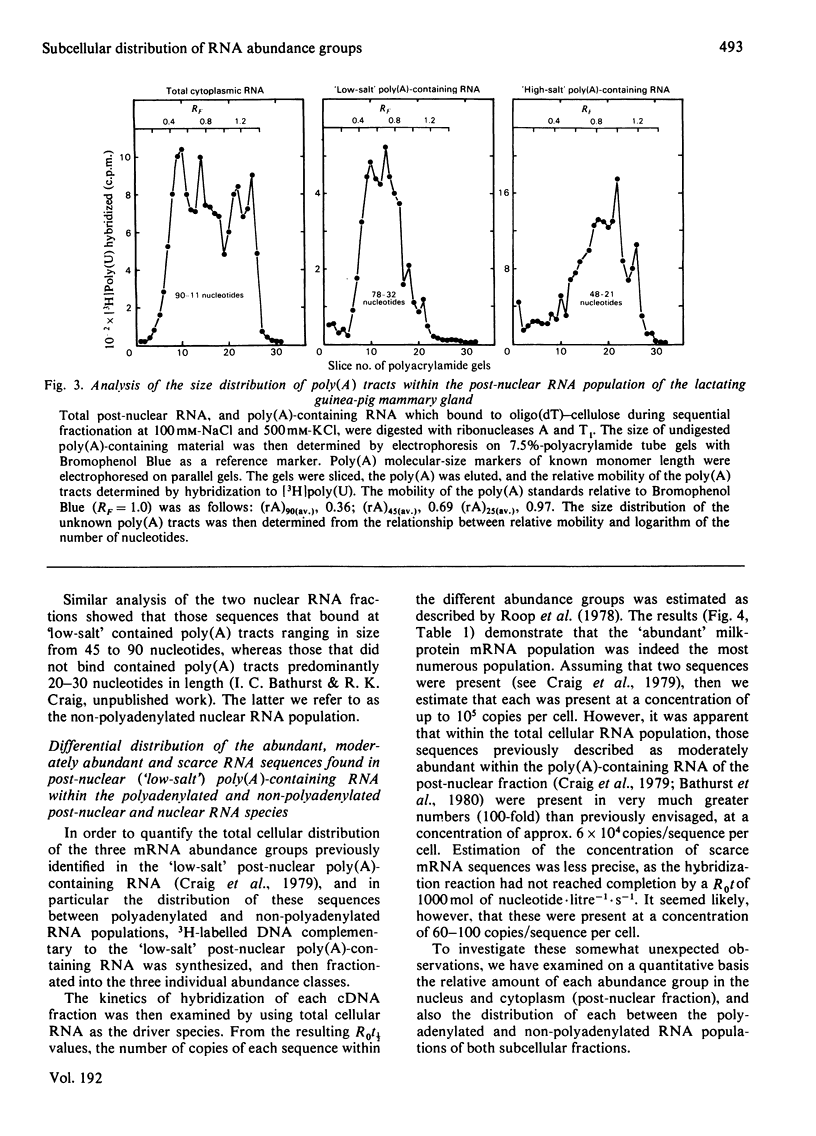
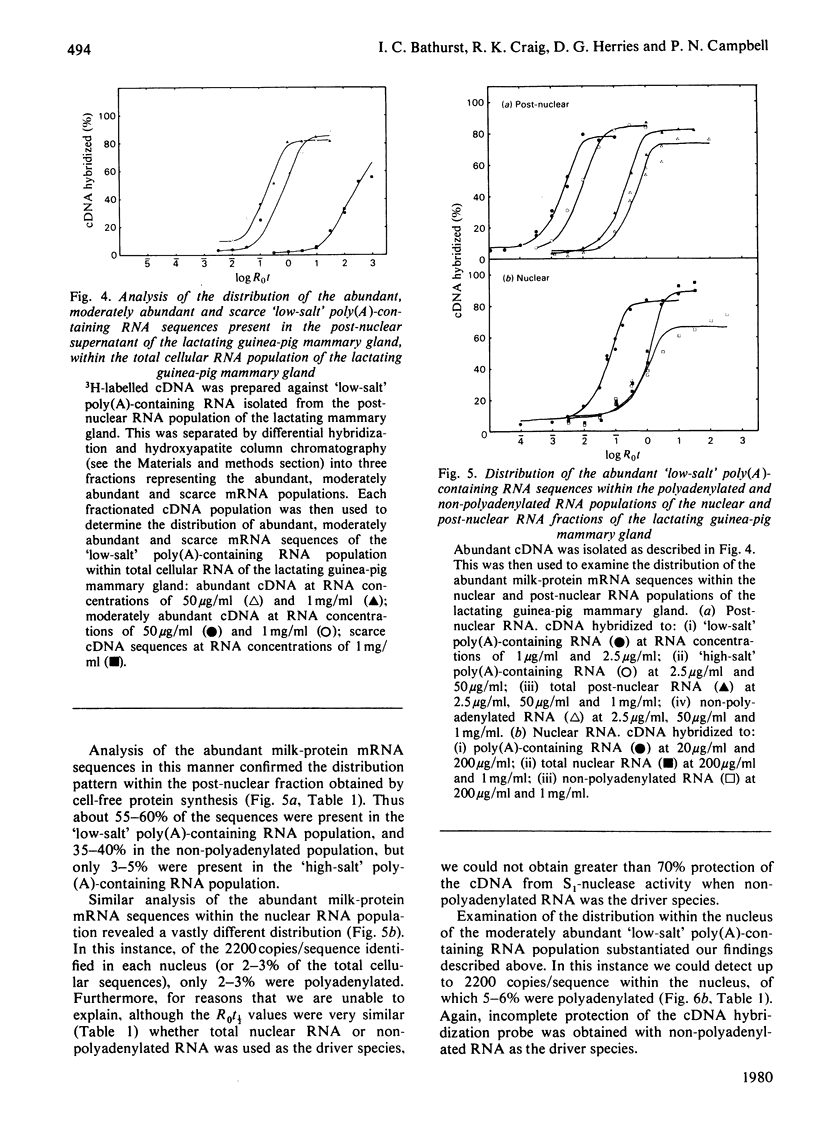
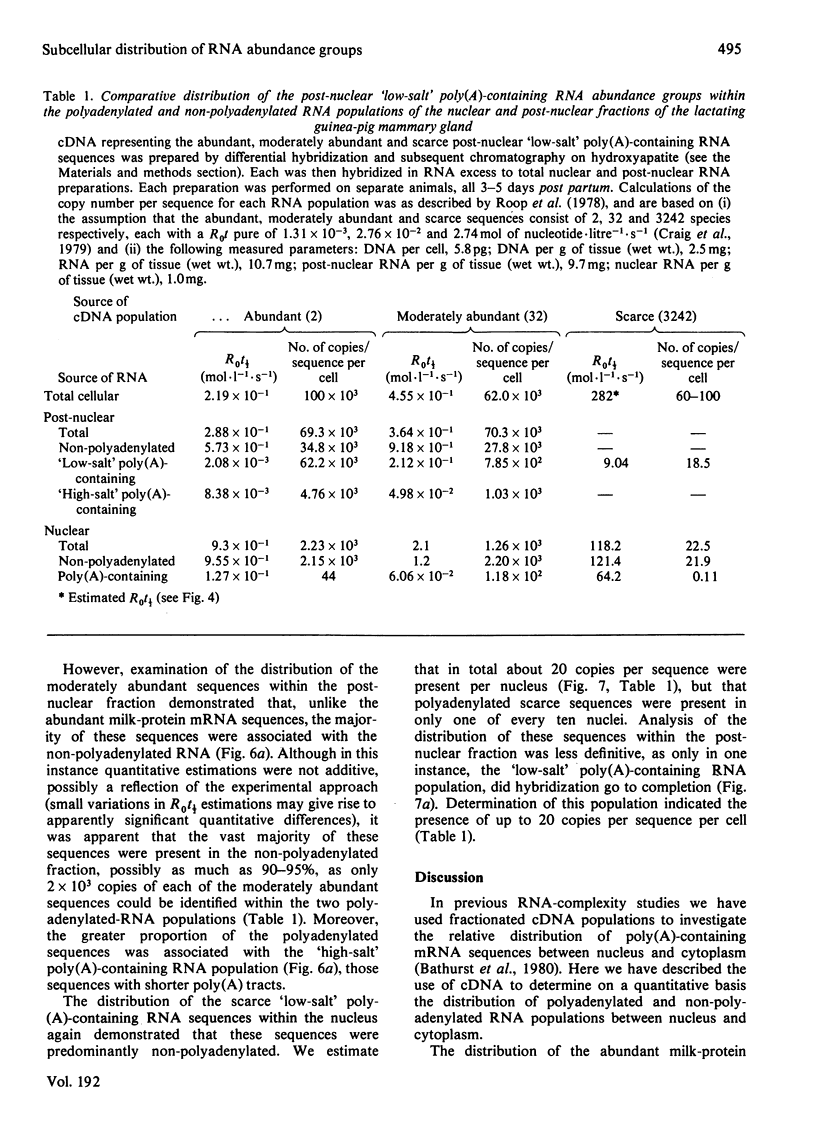
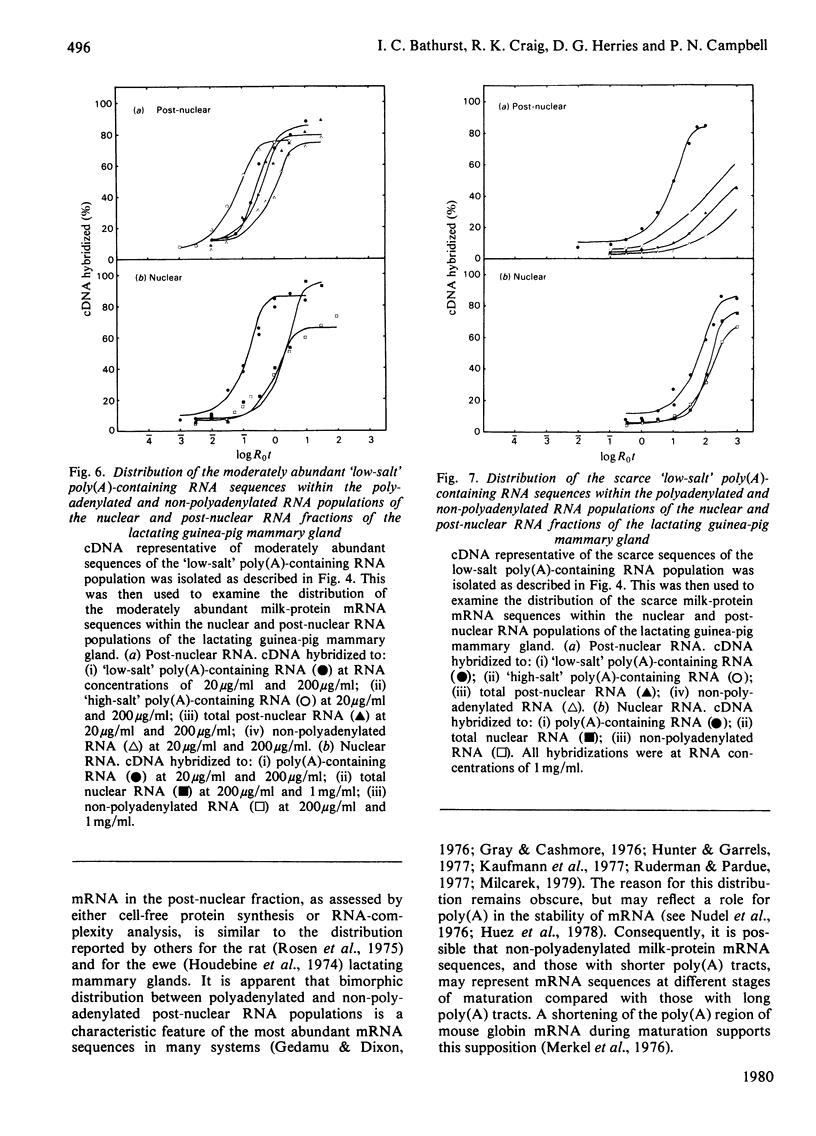
1. RNA isolated from the post-nuclear supernatant of the lactating guinea-pig mammary gland was fractionated with oligo(dT)–cellulose into three populations; those that bound at `low salt' [long poly(A) tracts, 78–32 nucleotides]; those that bound at `high salt' [shorter poly(A) tracts, 48–21 nucleotides]; and those that did not bind [no poly(A) or short poly(A) tracts, <20 nucleotides]. Nuclear RNA was fractionated into two populations, those that bound in `low salt' and those that did not bind. All the post-nuclear RNA fractions directed the synthesis of milk proteins in a Krebs II ascites cell-free system. 2. 3H-labelled DNA complementary to the post-nuclear poly-(A)-containing RNA population (low-salt fraction) was fractionated into abundant (milk-protein mRNA), moderately abundant and scarce sequences. This complementary DNA was then used to investigate the distribution of the mRNA sequences in the different RNA populations. This showed that all sequences were present in polyadenylated and non-polyadenylated fractions, but that major quantitative differences were apparent. The abundant milk-protein mRNA sequences predominated in the `low-salt' post-nuclear poly(A)-containing RNA fraction, whereas the moderately abundant sequences predominated in the non-polyadenylated post-nuclear RNA fraction. In total cellular RNA, those sequences deemed initially to be moderately abundant within the `low-salt' poly(A)-containing RNA population were present at a concentration very similar to those of the abundant milk-protein mRNA (approx. 6×105 copies of each sequence/cell). Similarly, analysis of the nuclear RNA populations showed that the `abundant' and so-called `moderately abundant' sequences were present in essentially identical concentrations (2×103 copies of each sequence/cell). The majority of these (90–95%) were non-polyadenylated. 3. The results are discussed in terms of the post-transcriptional mechanisms involved in the regulation of gene expression in the lactating guinea-pig mammary gland.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathurst I. C., Craig R. K., Herries D. G., Campbell P. N. Differential distribution of poly(A)-containing RNA sequences between the nucleus and post-nuclear supernatant of the lactating guinea-pig mammary gland. Eur J Biochem. 1980 Aug;109(1):183–191. doi: 10.1111/j.1432-1033.1980.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P., Verma D. P., Fromson D. Polyadenylated and nonpolyadenylated messenger RNA fractions from sea urchin embryos code for the same abundant proteins. Dev Biol. 1979 Jul;71(1):128–141. doi: 10.1016/0012-1606(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Chikaraishi D. M. Complexity of cytoplasmic polyadenylated and nonpolyadenylated rat brain ribonucleic acids. Biochemistry. 1979 Jul 24;18(15):3249–3256. doi: 10.1021/bi00582a009. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Boulton A. P., Harrison O. S., Parker D., Campbell P. N. Studies on the intracellular segregation of polyribosome-associated messenger ribonucleic acid species in the lactating guinea-pig mammary gland. Biochem J. 1979 Sep 1;181(3):737–756. doi: 10.1042/bj1810737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H. Purification and properties of biologically active rainbow trout testis protamine mRNA. J Biol Chem. 1976 Mar 10;251(5):1455–1463. [PubMed] [Google Scholar]
- Grady L. J., North A. B., Campbell W. P. Complexity of poly(A+) and poly(A-) polysomal RNA in mouse liver and cultured mouse fibroblasts. Nucleic Acids Res. 1978 Mar;5(3):697–712. doi: 10.1093/nar/5.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. E., Cashmore A. R. RNA synthesis in plant leaf tissue: the characterization of messenger RNA species lacking and containing polyadenylic acid. J Mol Biol. 1976 Dec 15;108(3):595–608. doi: 10.1016/s0022-2836(76)80139-8. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Campbell P. N. mRNA species directing synthesis of milk proteins in normal and tumour tissue from human mammary gland. Nature. 1979 Jan 4;277(5691):54–56. doi: 10.1038/277054a0. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M. Absence of poly (A) in a large part of newly synthesized casein mRNAs. FEBS Lett. 1976 Jul 1;66(1):110–113. doi: 10.1016/0014-5793(76)80597-2. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M., Gaye P., Favre A. Lack of poly(A) sequence in half of the messenger RNA coding for ewe alpha S casein. Nucleic Acids Res. 1974 Mar;1(3):413–426. doi: 10.1093/nar/1.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Gallwitz D., Weinberg E., Devos R., Hubert E., Cleuter Y. Functional stabilisation of HeLa cell histone messenger RNAs injected into Xenopus oocytes by 3'-OH polyadenylation. Nature. 1978 Feb 9;271(5645):572–573. doi: 10.1038/271572a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann Y., Milcarek C., Berissi H., Penman S. HeLa cell poly(A)- mRNA codes for a subset of poly(A)+ mRNA-directed proteins with an actin as a major product. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4801–4805. doi: 10.1073/pnas.74.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. A change in the stability of globin mRNA during the induction of murine erythroleukemia cells. Cell. 1978 Jun;14(2):337–344. doi: 10.1016/0092-8674(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Merkel C. G., Wood T. G., Lingrel J. B. Shortening of the poly(A) region of mouse globin messenger RNA. J Biol Chem. 1976 Sep 25;251(18):5512–5515. [PubMed] [Google Scholar]
- Milcarek C. HeLa cell cytoplasmic mRNA contains three classes of sequences: predominantly poly(A)-free, predominantly poly(A)-containing and bimorphic. Eur J Biochem. 1979 Dec 17;102(2):467–476. doi: 10.1111/j.1432-1033.1979.tb04261.x. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Miller L. Relative amounts of newly synthesized poly(A)+ and poly(A)- messenger RNA during development of Xenopus laevis. Dev Biol. 1978 May;64(1):118–129. doi: 10.1016/0012-1606(78)90064-7. [DOI] [PubMed] [Google Scholar]
- Nemer M., Graham M., Dubroff L. M. Co-existence of non-histone messenger RNA species lacking and containing polyadenylic acid in sea urchin embryos. J Mol Biol. 1974 Nov 5;89(3):435–454. doi: 10.1016/0022-2836(74)90474-4. [DOI] [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Ragg H., Schröder J., Hahlbrock K. Translation of poly(A)-containing and poly(A)-free messenger RNA for phenylalanine ammonia-lyase, a plant-specific protein, in a reticulocyte lysate. Biochim Biophys Acta. 1977 Jan 20;474(2):226–233. doi: 10.1016/0005-2787(77)90197-6. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Nordstrom J. L., Tsai S. Y., Tsai M. J., O'Malley B. W. Transcription of structural and intervening sequences in the ovalbumin gene and identification of potential ovalbumin mRNA precursors. Cell. 1978 Oct;15(2):671–685. doi: 10.1016/0092-8674(78)90035-1. [DOI] [PubMed] [Google Scholar]
- Rosbash M. Polyadenylic acid-containing RNA in Xenopus laevis oocytes. J Mol Biol. 1974 May 5;85(1):87–101. doi: 10.1016/0022-2836(74)90131-4. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Ruderman J. V., Pardue M. L. A portion of all major classes of histone messenger RNA in amphibian oocytes is polyadenylated. J Biol Chem. 1978 Mar 25;253(6):2018–2025. [PubMed] [Google Scholar]
- Ruderman J. V., Pardue M. L. Cell-free translation analysis of messenger RNA in echinoderm and amphibian early development. Dev Biol. 1977 Oct 1;60(1):48–68. doi: 10.1016/0012-1606(77)90109-9. [DOI] [PubMed] [Google Scholar]
- Spohr G., Dettori G., Manzari V. Globin mRNA sequences in polyadenylated and nonpolyadenylated nuclear precursor-messenger RNA from avian erythroblasts. Cell. 1976 Aug;8(4):505–512. doi: 10.1016/0092-8674(76)90218-x. [DOI] [PubMed] [Google Scholar]
- Van Ness J., Maxwell I. H., Hahn W. E. Complex population of nonpolyadenylated messenger RNA in mouse brain. Cell. 1979 Dec;18(4):1341–1349. doi: 10.1016/0092-8674(79)90244-7. [DOI] [PubMed] [Google Scholar]