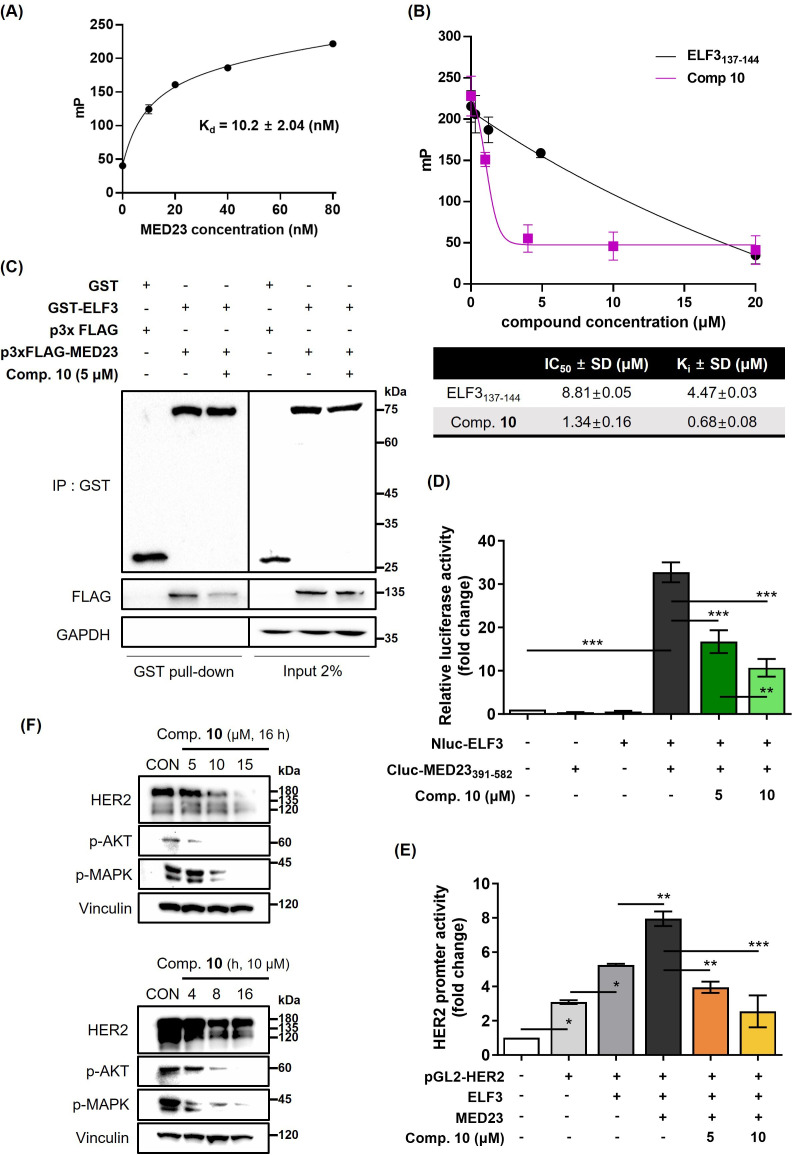
Figure 5. Compound 10 as a transcriptional regulator of HER2 by inhibiting ELF3-MED23 PPI.
(A) Titration curve of MED23391-582 protein FITC-labeled ELF3129-145 peptide. Binding of the MED23391-582 protein and ELF3-FITC peptide (17 a.a.) was validated via cell-free FP assay. Kd value was measured as 10.2±0.82 (nM) using the least squares non-linear fit method (n=3, mean ± S.D). (B) Effect of compound 10 on the FP (mP) induced by the binding of FITC-ELF3129-145 peptide to (His)6-MED23391-582 protein was evaluated in cell-free system. Unlabeled ELF3137-144 peptide was used as positive control. IC50 and Ki values were calculated from the FP assay results (n=3, mean ±S.D.). (C) Intracellular PPI inhibitory effect of compound 10 (5 μM, 12 h treatment) against ELF3-MED23 was evaluated through GST-pull down assay using GST-ELF3WT and 3xFLAG-MED23. (D) Impact of compound 10 on the relative luciferase activity generated by Nluc-ELF3WT and Cluc-MED23391-582 interaction was evaluated (20 hr treatment at indicated concentrations, n=3, mean ± S.D., ANOVA, ***p<0.001). (E) Effect of compound 10 on the overall ERBB2 promoter activity was assessed (20 hr treatment at indicated concentrations, n=3, mean ± S.D., ANOVA, *p<0.05, **p<0.01, and ***p<0.001). (F) Changes in the HER2 and its downstream signaling pathway were evaluated by treating compound 10 in dose- and time-dependent manner.