Abstract
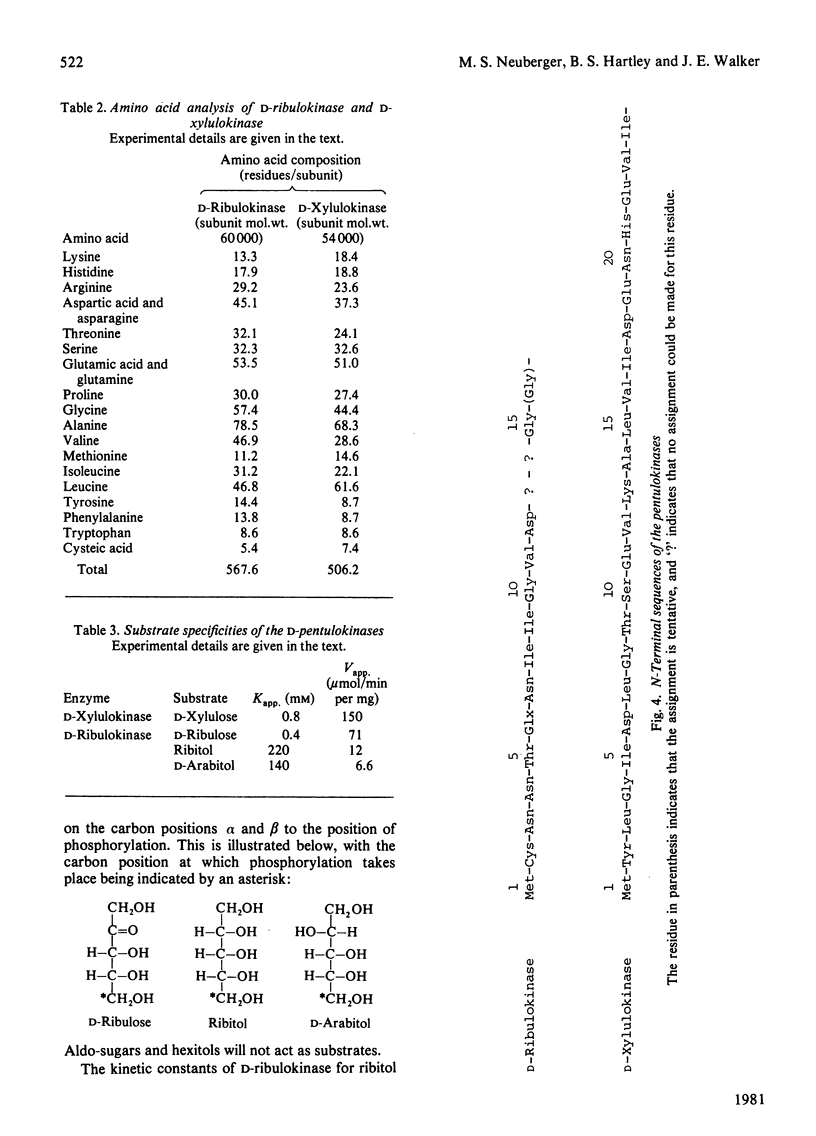
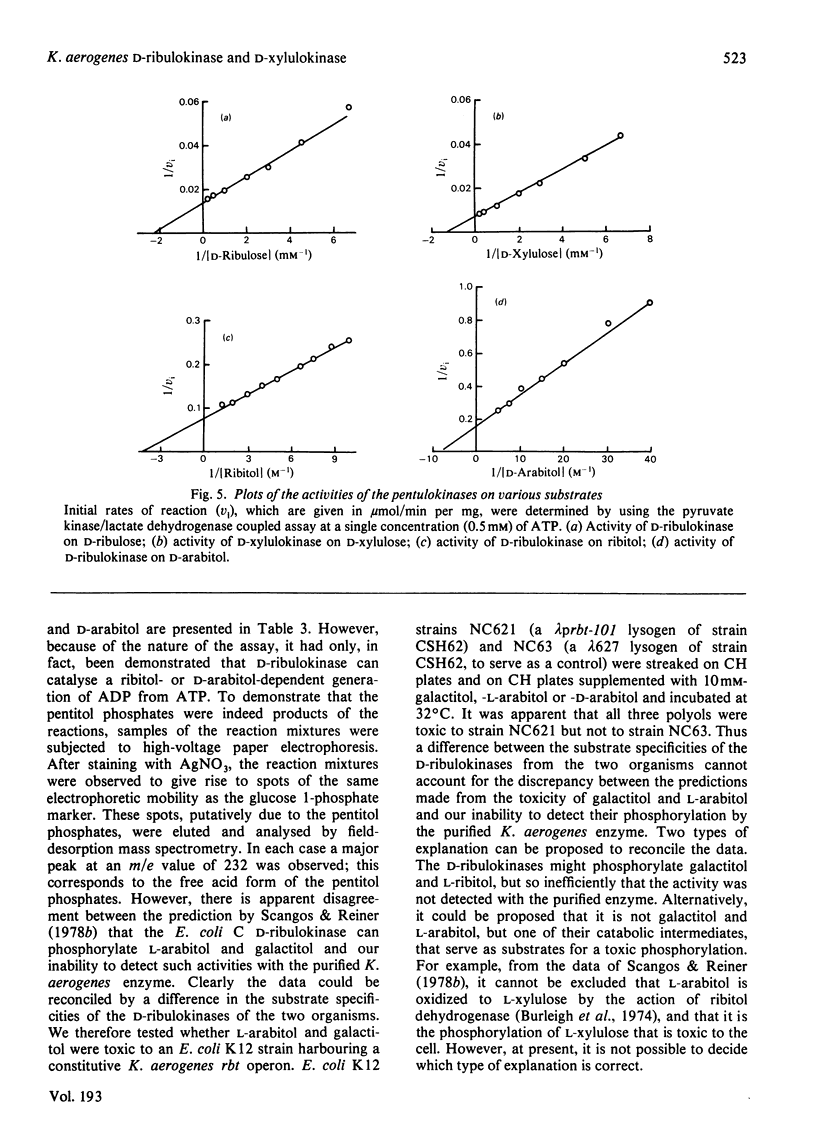
The D-ribulokinase and D-xylulokinase of Klebsiella aerogenes were purified to homogeneity from Escherichia coli K12 construct strains that synthesized these enzymes constitutively. The D-ribulokinase, which is encoded in the ribitol operon, is active as a dimer of 60 000 subunit mol.wt., whereas the D-xylulokinase, which is encoded in the D-arabitol operon, is active as a dimer of 54 000 subunit mol.wt. The amino acid compositions and N-terminal sequences of both pentulokinases are reported. The Kapp. values of the enzymes for their D-pentulose substrates were determined, and the D-ribulokinase was shown to have a low-affinity side-specificity for ribitol and D-arabitol. These results are discussed in the context of the evolution of the Klebsiella aerogenes pentitol operons.
Full text
PDF




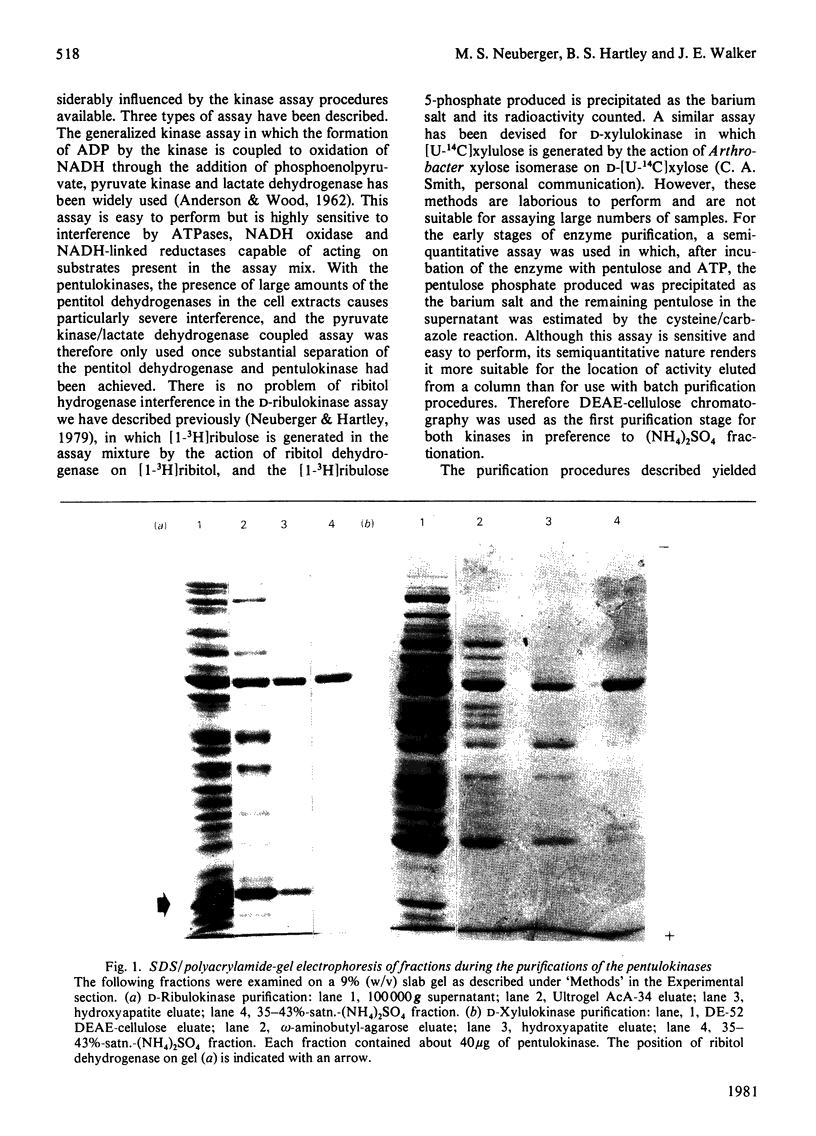

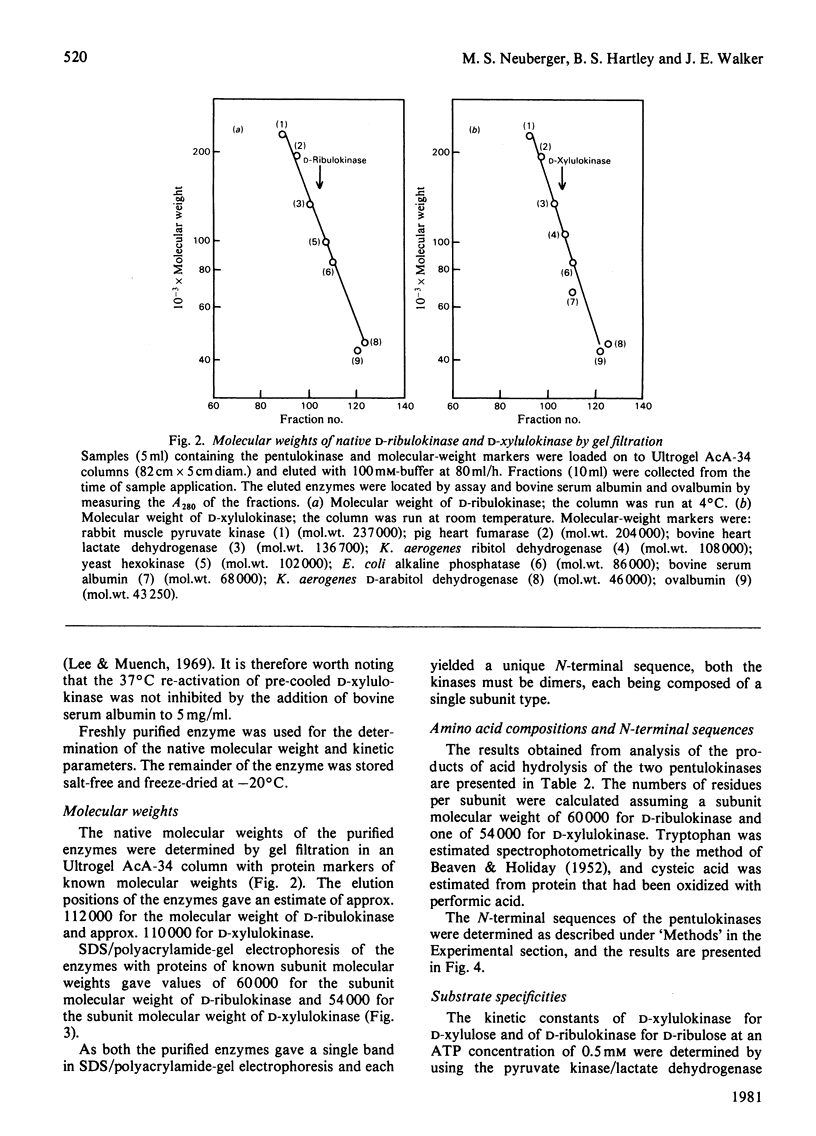
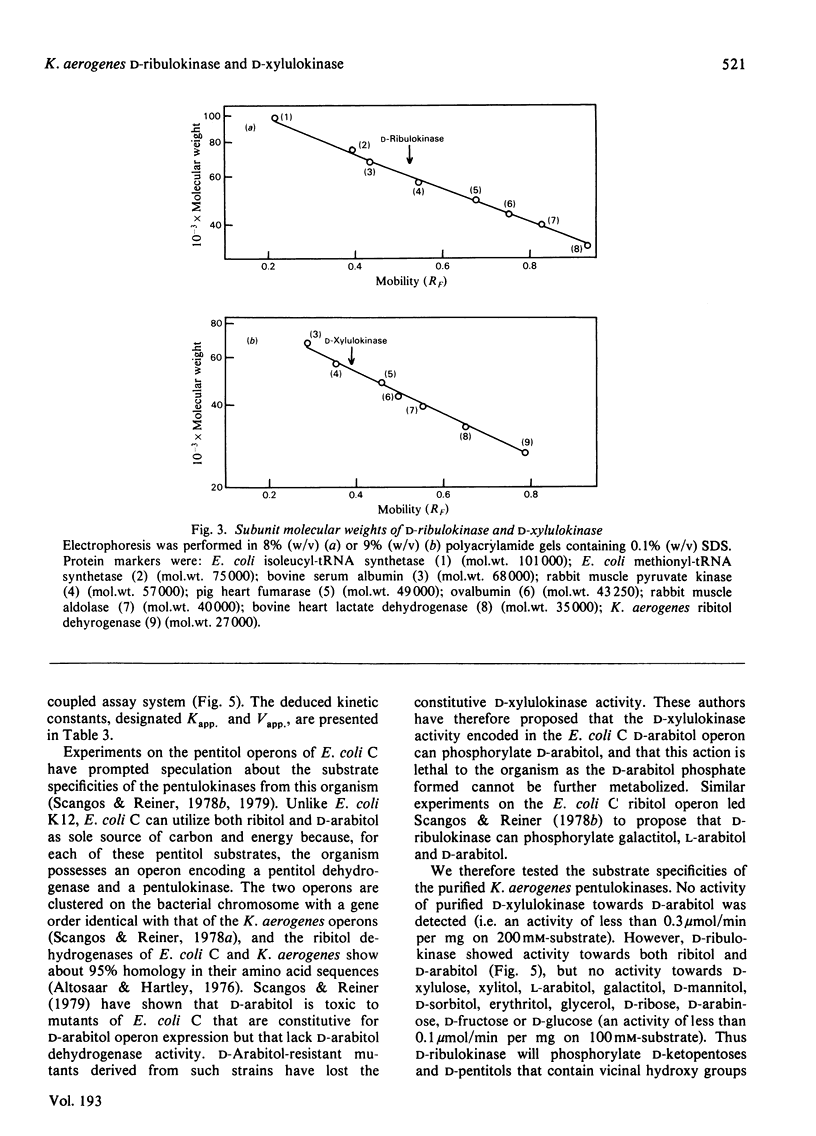

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON R. L., WOOD W. A. Purification and properties of L-xylulokinase. J Biol Chem. 1962 Apr;237:1029–1033. [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Burleigh B. D., Rigby P. W., Hartley B. S. A comparison of wild-type and mutant ribitol dehydrogenases from Klebsiella aerogenes. Biochem J. 1974 Nov;143(2):341–352. doi: 10.1042/bj1430341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Close genetic linkage of the determinants of the ribitol and D-arabitol catabolic pathways in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):176–182. doi: 10.1128/jb.119.1.176-182.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. M., Hartley B. S. Purification, properties and specificity of the restriction endonuclease from Bacillus stearothermophilus. Biochem J. 1979 Jan 1;177(1):49–62. doi: 10.1042/bj1770049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Tanaka T. Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies. J Biochem. 1972 Jun;71(6):1043–1051. doi: 10.1093/oxfordjournals.jbchem.a129852. [DOI] [PubMed] [Google Scholar]
- Lee M. L., Muench K. H. Prolyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification and evidence for subunits. J Biol Chem. 1969 Jan 25;244(2):223–230. [PubMed] [Google Scholar]
- Neuberger M. S., Hartley B. S. Investigations into the Klebsiella aerogenes pentitol operons using specialised transducing phages lambdaprbt and lambdaprbt dal. J Mol Biol. 1979 Aug 15;132(3):435–470. doi: 10.1016/0022-2836(79)90269-9. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Patterson R. A., Hartley B. S. Purification and properties of Klebsiella aerogenes D-arabitol dehydrogenase. Biochem J. 1979 Oct 1;183(1):31–42. doi: 10.1042/bj1830031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Scangos G. A., Reiner A. M. A unique pattern of toxic synthesis in pentitol catabolism: implications for evolution. J Mol Evol. 1979 Mar 15;12(3):189–195. doi: 10.1007/BF01732338. [DOI] [PubMed] [Google Scholar]
- Scangos G. A., Reiner A. M. Acquisition of ability to utilize Xylitol: disadvantages of a constitutive catabolic pathway in Escherichia coli. J Bacteriol. 1978 May;134(2):501–505. doi: 10.1128/jb.134.2.501-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G. A., Reiner A. M. Ribitol and D-arabitol catabolism in Escherichia coli. J Bacteriol. 1978 May;134(2):492–500. doi: 10.1128/jb.134.2.492-500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Rigby P. W., Hartley B. S. Ribitol dehydrogenase from Klebsiella aerogenes. Purification and subunit structure. Biochem J. 1974 Sep;141(3):693–700. doi: 10.1042/bj1410693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Carne A. F., Runswick M. J., Bridgen J., Harris J. I. D-glyceraldehyde-3-phosphate dehydrogenase. Complete amino-acid sequence of the enzyme from Bacillus stearothermophilus. Eur J Biochem. 1980 Jul;108(2):549–565. doi: 10.1111/j.1432-1033.1980.tb04751.x. [DOI] [PubMed] [Google Scholar]
- Wilson B. L., Mortlock R. P. Regulation of D-xylose and D-arabitol catabolism by Aerobacter aerogenes. J Bacteriol. 1973 Mar;113(3):1404–1411. doi: 10.1128/jb.113.3.1404-1411.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Lin E. C., Tanaka S. Mutants of Aerobacter aerogenes capable of utilizing xylitol as a novel carbon. J Bacteriol. 1968 Aug;96(2):447–456. doi: 10.1128/jb.96.2.447-456.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]