Abstract
Background
Patients who have experienced a cryptogenic stroke (CS) may benefit from extended monitoring and possible earlier detection of atrial fibrillation (AF), allowing for the timely initiation of appropriate pharmacotherapy.
Objective
This economic study aimed to evaluate the clinical and cost outcomes of using mid-term cardiac monitors (referred to as “ePatch”) versus ILR-only in post-CS patients in the UK, Netherlands (NL) and Sweden.
Methods
An existing cost-minimization model was modified to fit healthcare settings in the UK, Netherlands and Sweden. The model’s target population was composed of adult patients who had previously experienced a CS, but had no documented history of AF. The model compares the one-year direct medical costs between two groups: one group receiving wearable ePatch, the other group proceeding directly to ILR.
Results
When applied to a group of 1,000 patients, the ePatch versus ILR approach resulted in cost savings, due to combination of reduced expenses and decreased modelled occurrence of recurrent strokes in all three countries studied. In the base case analysis, the cost savings per patient with detected AF for ePatch ranged from 3.4–6.0 times, depending on the country.
Conclusion
Utilizing ePatch extended wear Holter for mid-term ECG monitoring in CS patients represents a cost-saving alternative to monitoring with ILR. The cost savings were achieved by reducing device expenses and by prevention of recurrent strokes via earlier anticoagulation initiation. Preventing recurrent strokes in this population is highly significant, as it can lead to improved long-term health outcomes and reduced overall healthcare costs.
Keywords: outpatient cardiac monitoring, stroke, holter, atrial fibrillation, electrocardiography, economic evaluation, secondary prevention, telemedicine, remote monitoring
Introduction
Stroke is the third leading cause of early mortality in Europe and a significant contributor to prolonged, yet preventable, disability in adults. Costs associated with disability due to stroke introduces a considerable societal burden.1 Every year, about 1.1 million people in the European Union suffer from stroke, with 440,000 patients succumbing to the condition. In 2015, the direct and indirect costs of stroke care and associated productivity loss amounted to €45 billion in the EU, making it a major public health concern.2
Stroke is typically classified into two major subtypes: hemorrhagic (~10-20% of stroke patients), and ischemic (~ 80–90% of cases).3 The results of the Oxfordshire Community Stroke project indicated that stroke has a recurrence rate of ~30% at 5 years. The risk of stroke recurrence and death is highest in the first year post-stroke.4–8
Approximately 25–30% of ischemic strokes are considered cryptogenic.9–16 One possible etiology is cardiac embolism secondary to occult paroxysmal atrial fibrillation (AF).17–19
A diagnosis of AF, in and of itself, confers a five to sixfold increased risk of ischemic stroke,20–25 risk which can be significantly reduced (by up to two-thirds) with anticoagulant (OAC)/antiplatelet therapy.26–28 While not specifically directed towards post-stroke patients, the NOAH AFNET and ARTESIA trials, both undertaken in patients with implantable cardiac devices and subclinical atrial fibrillation, with some controversy, have demonstrated stroke reduction with the initiation of anticoagulation, albeit at the expense of bleeding.29 In stroke patients, guidelines recommend a confirmed diagnosis of AF to initiate OAC therapy,30 whereas in the absence of AF antiplatelet therapy is usually recommended to patients who have suffered acute ischemic stroke.31
Diagnosing paroxysmal AF can be challenging, as an episode may not be observed during short-term electrographic monitoring. Additionally, asymptomatic AF goes undetected unless incidentally discovered on electrocardiogram (ECG) or via telemetry monitoring. If AF is suspected, the likelihood of detecting asymptomatic paroxysmal AF increases with the duration of monitoring or with repeated monitoring strategies.32,33
Several short-, middle-, and long-term monitoring strategies exist for the detection of AF in stroke patients.34 Commonly used cardiac monitoring methods typically include Holter monitors for short-term periods (24–48 hours), extend wear Holter recordings for mid-term periods (up to 14 days), and long-term recorders (up to 30 days) like post-event recorders (non-looping recorders), external loop recorders (ELR), mobile cardiac outpatient telemetry (MCOT) and implantable loop recorders (ILR).35
The ePatch (Philips Healthcare, Netherlands) is a digital extended wear Holter delivering comprehensive arrhythmic reports for adults aged 18 and older who may be asymptomatic or experience transient clinical symptoms. A sensor is connected to a compatible ECG electrode (patch) and placed on the patient’s upper sternum or upper torso. The ePatch sensor is configured for 1, 2, or 3 channels of ECG for up to 16 continuous days and patients can trigger recording of symptomatic events. At the end of the recording, data is uploaded via a computer’s USB cable and transferred for Holter analysis by qualified healthcare professionals.
Implantable loop recorders are small, subcutaneously implanted devices used for long-term cardiac monitoring. ILRs continuously record cardiac electrical activity and can store data automatically when arrhythmias are detected or can be triggered manually by the patient during symptomatic episodes. Stored data is transmitted to healthcare providers, either remotely or during follow-up visits, for analysis and diagnosis. ILRs are used for diagnosing intermittent, unexplained symptoms and offer detection of arrhythmias over extended periods.
A large systematic literature review and network meta-analysis by Sposato et al35 demonstrated that mid-term monitoring over 21 days is significantly more effective in detecting post-stroke AF than long-term monitoring also at 21 days, with an adjusted hazard ratio of 5.8 (95% CI 3.3–10.2; p<0.0001). While the difference in AF detection rates between mid-term and long-term monitoring is slightly lower at 14 days, it remains statistically significant, highlighting the benefit of early, intensive AF monitoring in cryptogenic stroke patients.
The European guidelines for diagnosing and managing AF advocate for utilization of more advanced and extended monitoring techniques, as they may enhance the detection of AF.36 The cost-effectiveness of mid-term monitoring compared to ILR in AF screening in the post-stroke setting remains unknown. An economic evaluation comparing these two options may inform decision-makers about clinical outcomes and financial implications, thus enabling well-informed choices.
The objective of this study was to evaluate the cost and clinical outcomes associated with the use of an outpatient middle-term monitoring strategy, specifically, the use of a 14-day ePatch (Philips Healthcare, Netherlands) in the adult post-stroke population. This strategy was directly compared to ILR worn for 6 months.
Materials and Methods
Model Design
This economic analysis aimed to assess costs associated with early use of 14 days of ePatch monitoring, compared to early implantation of ILR in cryptogenic stroke patients from the perspective of three European countries: the United Kingdom (UK), the Netherlands (NL), and Sweden (SW). We analyzed the “net cost” for each ECG modality, specifically considering the cost per patient monitored and the cost per patient with detected AF.
The constructed model followed a similar structure as one developed by Medic et al (2021),37 which compared the costs and outcomes of cardiac rhythm monitoring modalities from the perspective of US healthcare payers. Many elements of the US model such as concepts, fundamental modeling approach, and health state definitions were inherited, while the comparisons, modeling structure, the cost inputs and scenarios were adjusted for the respective European countries.
In the UK, clinical practice based on NICE guidelines38 advises that cryptogenic stroke patients initially undergo non-invasive ECG monitoring before ILR implantation is considered.38 This approach is recommended as AF in these patients may be intermittent, brief, and asymptomatic, making detection challenging. Extended monitoring has been found to reveal AF in around one in six cryptogenic stroke patients.39 UK guidelines align closely with European Guidelines,36 providing a foundation for this model’s development. Similarly, Dutch guidelines40 are consistent with these standards. In contrast, clinical practice for AF screening following ischemic stroke or transient ischemic attack (TIA) varies across Swedish stroke units, with differences in both AF screening methods and monitoring durations.41 ILR is recognized as a potential tool for detecting atrial fibrillation in patients with cryptogenic stroke in Sweden; however, the scientific evidence supporting its effectiveness in this context remains limited.42 Consequently, a reimbursement pathway for ILR in post-stroke patients has not been established in Sweden. While guidelines and clinical practice recommendations provide a structured approach, managing and monitoring AF in real-life clinical settings requires a more individualized approach. Each patient presents unique characteristics that may influence AF detection, including variations in heart rhythm, the likelihood of asymptomatic episodes, and differences in comorbidities that affect monitoring needs. Thus, diagnosing AF in a specific patient often demands an adaptable approach, as what proves effective for one patient may not be suitable for another. For example, some patients may benefit from prolonged non-invasive monitoring, while others may require early ILR implantation for effective AF detection. This variability highlights the importance of personalized diagnostic strategies, enabling clinicians to tailor monitoring methods to each patient’s specific clinical profile and improving the chances of timely and accurate AF diagnosis.
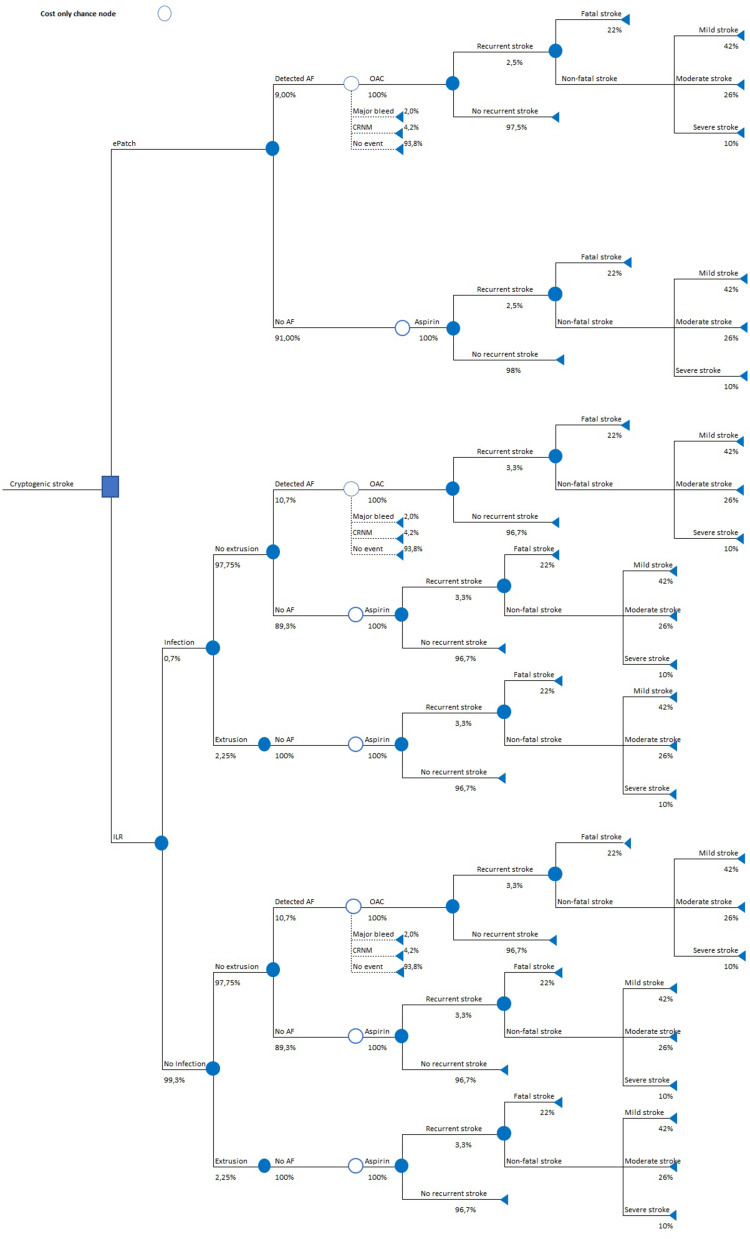
The original publication37 discussed the modeling approach in detail. In brief, the cost-minimization model employed the decision-tree framework applying a one-year time horizon without discounting costs or outcomes. Figure 1 illustrates the model structure used for quantifying costs and outcomes at every stage of monitoring and treatment. The model included two diagnostic and monitoring arms to be used immediately following cryptogenic stroke:
ePatch arm: use of 14-day ePatch to detect AF;
ILR arm: use of ILR to detect AF in the first 6 months post cryptogenic stroke.
Figure 1.
Model structure.
Abbreviations: AF, atrial fibrillation; CRNM, clinically relevant non-major; ILR, implantable loop recorder; OAC, oral anticoagulant.
Perspective and Time Horizon
The economic assessments were taken from health care payer’s perspective of each country (NHS and Personal Social Services in the UK; the Ministry of Health, Welfare, and Sport in the Netherlands; and the Dental and Pharmaceutical Benefits Agency in Sweden) over a one-year time horizon. The model only considered direct medical costs.
Input Parameters
Costs
Unit costs obtained from published literature prior to 2022 were adjusted using the recommended43–45 country-specific inflation indices.46–48 The cost inputs and results were reported in Euros (2022) and local currencies (in the case of the UK and Sweden). Swedish krona (SEK) and UK pounds (£) were converted to EUR using an exchange rate of 11.1702 and 0.88348, respectively. An overview of all cost inputs used in the model is provided in Table 1.
Table 1.
Model Input Parameters – Costs
| Parameter | UK | Netherlands | Sweden |
|---|---|---|---|
| Drug costs (1-month therapy) | |||
| Aspirin | €9 (£8) | €11 a | €4 (kr39) |
| Dabigatran | €59 (£52) | €115 a | €60 (kr666) |
| Rivaroxaban | €62 (£55) | €66 a | €57 (kr635) |
| Apixaban | €66 (£58) | €68 a | €60 (kr668) |
| Edoxaban | €60 (£53) | €66 a | €60 (kr665) |
| Stroke costs | |||
| Recurrent mild stroke | €5,117 (£4,521) | €19,775 | €12,964 (kr144,815) |
| Recurrent moderate stroke | €26,699 (£23,588) | €54,841 | €26,039 (kr290,859) |
| Recurrent severe stroke | €36,466 (£32,217) | €67,173 | €32,618 (kr364,355) |
| Recurrent fatal stroke/death | €4,603 (£4,067) | €3,671 | €17,892 (kr199,856) |
| Cost of infection | |||
| Cost for superficial surgical site infections | €2,264 (£2,000) b | €2,000 b | €327 (kr3,655) c |
| Bleeding cost | |||
| Major bleeding | €4,940 (£4,365) d | €6,136 | €2,870 (kr32,058) |
| CRNM bleed event | €2,502 (£2,210) e | €40 | €910 (kr10,170) |
| ePatch costs | |||
| Device cost | €170 (£150) f | €140 f | €170 (kr1,899) f |
| ILR costs | |||
| Unit cost per ILR implantation | €600 (£530) g | €4,612 h | €2,285 (kr25,521) i |
| Unit cost per ILR device | €2,037 (£1,800) | NA | NA |
| Cost of daily continuous monitoring | €1,358 (£1,200) | NA | NA |
| Removal of ILR | €158 (£140) j | €2,463 k | NA |
Notes: a inclusive €6 delivery costs of the pharmacy; b Assumption; c NordDRG tariff E26O (cost was inclusive of ILR removal); d Weighted average of HRGs (FD03/HC28/HD24/BZ24/FF50/FF51), non elective long stay; e Weighted average of HRGs (FD03H/CA23/LB38H), non elective long stay; f Mean cost provided by the manufacturer. Mean cost provided is intended for modeling purposes only. The final actual cost may be higher or lower depending on various factors, including the specific service model chosen for ePatch, number of patients served or volume committed, and country-specific conditions and regulations. These variables can impact the overall cost; g Outpatient Procedures (OPROC). Service code 320. Cardiology. HRG EY12B; h Dutch tariff declaration code 15B776; i - NordDRG tariff E26O (cost was inclusive of ILR removal); j Outpatient Procedures (OPROC). Service code 320. Cardiology. HRG EY13Z; k Dutch tariff declaration code 15B778.
Abbreviations: CRNM, clinically relevant non-major; HRG, Healthcare Resource Groups; ICH, intracranial hemorrhage; ILR, implantable loop recorder; UK, United Kingdom.
Device Costs
The device costs of ePatch used in the model for the UK, Netherlands, and Sweden were €170 (£150), €140, and €170 (kr1,899), respectively. These costs were mean costs supplied by the manufacturer. It was assumed that the mean cost of ePatch entails 14 days of wear time inclusive of staff costs (reading and reporting). Mean price of ePatch provided is intended for modeling purposes only, the final actual price may be higher or lower depending on various factors, including the specific service model chosen for ePatch, number of patients served or volume committed, and country-specific conditions and regulations.
The costs associated with ILR varied depending on the country context. They could include the acquisition cost of the device,38 one-off costs for its insertion and later removal,49 patient monitoring,50 or constitute a single diagnosis-related group (DRG) cost.51,52
Acute and Chronic Care Costs of Atrial Fibrillation and Anticoagulant-Related Events
In the model, acute management costs for mild, moderate, severe, and fatal ischemic stroke were considered. The costs of ischemic stroke management were derived from published literature sources.
The stroke costs for the UK were derived from a population study, which estimated the acute and long-term costs of stroke in AF patients.53 Dutch data was based on a study that used data from a prospective non-randomized controlled cluster trial that compared stroke services.54 The Swedish values were sourced from research conducted on patient-level data on patients suffering from stroke, identified in patient administrative systems (PAS) from seven Swedish regions.55
Pharmacotherapy Costs
The model conservatively assumed that all patients began treatment with aspirin and only initiated treatment with OACs upon the detection of AF. Patients without AF continued taking aspirin. As per the original publication, the OACs considered in the model were dabigatran, rivaroxaban, apixaban, and edoxaban. Drug dosages were sourced from the relevant summary of product characteristics (SPCs).
As all drugs were taken orally by patients, it was assumed there were no administration or monitoring costs. The pharmacotherapy costs were obtained for the smallest generic pack size. The resulting price for the OAC treatment was an average monthly price for all four drugs.
To comply with published health economic guidelines in all three countries, the analysis used pharmacy-selling prices excluding value-added tax, as these were considered the most accurate reflection of the costs borne by the healthcare payer.56–58 The expenses associated with aspirin and OAC were computed on an annual basis, irrespective of whether a patient used them for the entire year, half a year, or any other duration.
Cost of Adverse Events and Complications
The model assumed that ILR implantation may be associated with the cost of managing a local non-fatal infection process. For the Swedish analysis, a valid DRG tariff51 was identified. For the UK and Dutch adaptation, an assumption was made to use a one-off cost of 2,000 (local currency).
The use of OACs may be associated with the development of bleeding complications. These were costed based on literature sources43,54,59 and country-level schedules of DRG tariffs.52
Event Probabilities
The probabilities of clinical events are reported in Table 2. The time point of 14 days was selected as that corresponds to a typical ePatch wear time. In each diagnostic modality, patients diagnosed with AF began appropriate OAC therapy. Undiagnosed patients continued taking aspirin alone (Figure 1).
Table 2.
Model Input Parameters - Event Probabilities
| Parameter | Value used in the model | Reference |
|---|---|---|
| Stroke-related probabilities | ||
| Recurrent mild stroke | 42% | [60] |
| Recurrent moderate stroke | 26% | [60] |
| Recurrent severe stroke | 10% | [60] |
| Recurrent fatal stroke/death | 22% | [60] |
| Base-case | ||
| Recurrent stroke in 1st year after ePatch | 2.5% | FIND-AF trial7 |
| No stroke in 1st year after ePatch | 97.5% | Calculation based on FIND-AF trial7 |
| Recurrent stroke in 1st year after ILR | 3.3% | PER DIEM trial61 |
| No stroke in 1st year after ILR | 96.7% | Calculation based on PER DIEM trial61 |
| Scenario 1 and 2 | ||
| Recurrent stroke in 1st year after ePatch | 6.4% | MonDAFIS trial62 |
| No stroke in 1st year after ePatch | 93.6% | Calculation based on MonDAFIS trial62 |
| Recurrent stroke in 1st year after ILR | 7.1% | PER DIEM trial16 |
| No stroke in 1st year after ILR | 92.9% | Calculation based on PER DIEM trial16 |
| Infection rate ILR | ||
| Infection rate after ILR | 0.7% | [63] |
| Extrusion rate ILR | ||
| Extrusion rate after ILR | 2.25% | [63] |
| Bleedings | ||
| Major bleeding | 2.02% | [64] |
| CRNM bleed event | 4.18% | [64] |
| Detection rates – Base case | ||
| Detected AF ePatch | 9.0% | FIND-AF7 |
| No AF [ePatch] | 91.0% | Calculation based on FIND-AF RCT7 |
| Detected AF ILR | 10.7% | PER DIEM trial61 |
| No AF [ILR] | 89.3% | Calculation based on PER DIEM trial61 |
| No AF [ILR] - After extrusion | 100.0% | Assumption |
| Scenario 1 | ||
| Detected AF ePatch | 5.8% | MonDAFIS trial62 |
| No AF [ePatch] | 94.2% | Calculation based on MonDAFIS trial62 |
| Detected AF ILR | 8.9% | CRYSTAL AF (6 months)16 |
| No AF [ILR] | 91.1% | Calculation based on CRYSTAL AF (6 months)16 |
| No AF [ILR] - After extrusion | 100.0% | Assumption |
| Scenario 2 | ||
| Detected AF ePatch | 9.8% | AF SPICE65 |
| No AF [ePatch] | 90.2% | Calculation based on AF SPICE65 |
| Detected AF ILR | 10.7% | PER DIEM trial61 |
| No AF [ILR] | 89.3% | Calculation based on PER DIEM trial61 |
| No AF [ILR] - After extrusion | 100.0% | Assumption |
| Drug usage probabilities | ||
| OAC usage post-detection of AF (base case) | 100% | Assumption |
| Aspirin usage (base case) | 100% | Assumption |
Abbreviations: AF = atrial fibrillation; CRNM = clinically relevant non-major; ILR = implantable loop recorder; OAC = oral anticoagulant.
In the base case, the incidence of recurring strokes within the first year varied depending on the monitoring method (Table 2). In the context of scenario analyses, the occurrence of recurrent strokes in the first year was contingent upon the monitoring modality (Table 2).
To account for variations in stroke outcomes due to severity, patients were stratified according to the Modified Rankin Scale (mRS) into ‘mild’, ‘moderate’, or ‘severe’ post-stroke states. A score of 0–2 on the mRS was defined as ‘mild’, while a score of 3–4 was defined as ‘moderate’. A score of 5 indicated a ‘severe’ case, and patients could also experience a fatal stroke.
Patients taking OACs could experience side effects, including major bleeding and clinically relevant non-major (CRNM) bleeds.
Analyses
In the base case, the incidence of recurring strokes within the first year varied depending on the monitoring method. The base case analysis summarized cumulative one-year costs that reflect an increment in the costs between ePatch and ILR.
Parameter uncertainty was assessed using a deterministic one-way sensitivity analysis (OWSA).66 Major clinical input parameters were individually varied by ±25% and the results were presented in a graph format.
Next to base case, authors also explored two scenarios where detection of AF and recurrent stroke rates in the first year varied per monitoring modality.
Model Outcomes
The primary outcome of the model was the difference in total costs between the ePatch and ILR-only groups for a cohort of 1,000 patients. Relevant secondary outcomes included: differences in costs per AF detected, the average cost per one patient monitored, incremental recurrent strokes avoided, and incremental infections avoided using ePatch vs ILR-only arms.
Results
Base Case results
The results of the base case economic analysis (per diagnosis arm and incremental results) are displayed in Tables 3 and 4.
Table 3.
Base Case - Clinical Results for a Cohort of 1,000 Patients – Different Monitoring Modalities – UK, Sweden and the Netherlands
| ePatch | ILR-only | Difference ePatch – ILR-only | |
|---|---|---|---|
| Number of patients with detected AF in a cohort of 1000 patients | 90 | 105 | −15 |
| Strokes | |||
| Number of recurrent mild strokes | 10.5 | 13.9 | −3.4 |
| Number of recurrent moderate strokes | 6.5 | 8.6 | −2.1 |
| Number of recurrent severe strokes | 2.5 | 3.3 | −0.8 |
| Number of recurrent fatal strokes/deaths | 5.5 | 7.3 | −1.8 |
| Total number of recurrent strokes | 25.0 | 33.0 | −8.0 |
| Bleedings | |||
| Number of major bleedings | 1.8 | 2.1 | −0.3 |
| Number of CRNM bleed events | 3.8 | 4.4 | −0.6 |
| Total number of bleedings | 5.6 | 6.5 | −0.9 |
| Infections/extrusions | |||
| Number of infections | 0.0 | 7.0 | −7.0 |
| Number of extrusions | 0.0 | 22.5 | −22.5 |
Notes: Percentage of undetected AF patients in ePatch group receive ILR for diagnosis.
Abbreviations: AF, atrial fibrillation; CRNM, clinically relevant non-major; ILR, implantable loop recorder.
Table 4.
Base Case - Economic Results for a Cohort of 1,000 Patients – Different Monitoring Modalities
| UK | Netherlands | Sweden | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ePatch | ILR-only | Difference ePatch – ILR-only | ePatch | ILR-only | Difference ePatch – ILR-only | ePatch | ILR-only | Difference ePatch – ILR-only | |
| Device | €170,000 (£150,000) | €4,153,752 (£3,669,757) | -€3,983,752 (-£3,519,757) |
€ 140,000 | € 7,074,670 | -€ 6,934,670 | €170,000 (kr 1,899,000) |
€2,284,771 (kr 25,521,352) |
-€2,114,771 (-kr 23,622,352) |
| Fatal strokes/Death | €25,319 (£22,369) |
€33,421 (£29,526) | -€8,102 (-£7,158) |
€ 18,059 | € 23,837 | -€ 5,779 | €98,405 (kr 1,099,206) |
€129,895 (kr 1,450,952) |
-€31,490 (-kr 351,746) |
| Recurrent strokes | €318,442 (£281,337) | €420,343 (£371,365) | -€101,901 (£90,028) |
€ 930,766 | € 1,228,611 | -€ 297,845 | €386,925 (kr 4,322,028) |
€510,741 (kr 5,705,077) |
-€123,816 (-kr 1,383,049) |
| Infections | €0 (£0) |
€15,846 (£14,000) | -€15,846 (-£14,000) |
€ 0 | € 14,000 | -€ 14,000 | €0 (kr 0) |
€2,291 (kr 25,585) |
-€2,291 (-kr 25,585) |
| Bleeding events | €18,394 (£16,251) |
€21,376 (£18,885) |
-€2,982 (-£2,635) |
€ 37,859 | € 43,998 | -€ 6,138 | €8,643 (kr 96,540) |
€10,044 (kr 112,193) |
-€1,401 (-kr 15,653) |
| Aspirin costs | €94,247 (£83,265) | €92,735 (£81,930) | €1,511 (£1,335) |
€ 125,471 | € 123,459 | € 2,012 | €38,309 (kr 427,915) |
€37,694 (kr 421,054) |
€614 (kr 6,862) |
| OAC costs | €66,646 (£58,880) | €77,452 (£68,427) | -€10,806 (-£9,547) |
€ 91,383 | € 106,200 | -€ 14,817 | €63,667 (kr 711,175) |
€73,990 (kr 826,485) |
-€10,323 (-kr 115,309) |
| Total costs | €693,047 (£612,101) | €4,814,926 (£4,253,890) |
-€4,121,879 (-£3,641,789) |
€ 1,343,537 | € 8,614,774 | -€ 7,271,237 |
€765,949 (kr 8,555,865) |
€3,049,426 (kr 34,062,698) |
-€2,283,477 (-kr 25,506,833) |
| Cost per patient with detected AF | €7,701 (£6,801) |
€46,035 (£40,671) | -€38,335 (-£33,870) |
€ 14,928 | € 82,365 | -€ 67,437 | €8,511 (kr 96,065) |
€29,155 (kr 325,671) |
-€20,645 (-kr 230,605) |
| Cost per 1 patient monitored | €693 (£612) |
€4,815 (£4,254) |
-€4,122 (-£3,642) |
€ 1,344 | € 8,615 | -€ 7,271 | €766 (kr 8,556) |
€3,049 (kr 34,063) |
-€2,283 (-kr 25,507) |
Note: Percentage of undetected AF patients in ePatch group receive ILR for diagnosis.
Abbreviations: AF, atrial fibrillation; ILR, implantable loop recorder; OAC, oral anticoagulant.
The ILR arm detected 16.67% more patients with AF compared to the ePatch arm; based on a cohort of 1,000 patients (105 vs 90 patients with detected AF, respectively). A higher number of strokes and bleeding events occurred in the ILR arm compared to ePatch arm (33 vs 25 total number of recurring strokes; 6.5 vs 5.6 bleeding events, respectively, in a cohort of 1,000). Additionally, 1.8 fatal strokes were avoided in a cohort of 1,000 patients annually for those diagnosed with ePatch compared to ILR-only.
Using ePatch for diagnosing AF after cryptogenic stroke leads to significant cost savings compared to ILR in all three countries. Additionally, the cost per patient with detected AF and the cost per monitored patient were lower in the ePatch arm than in the ILR arm (Table 4).
Model Uncertainty
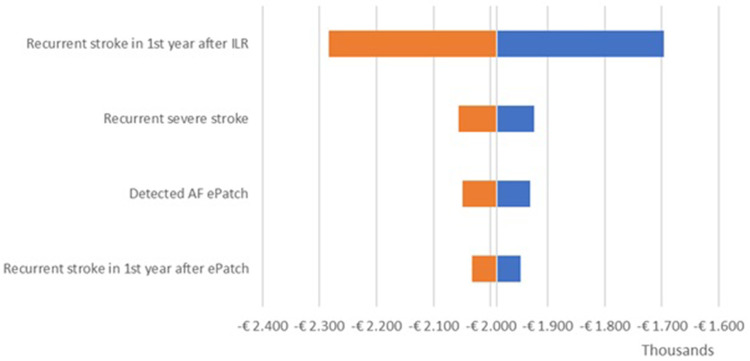
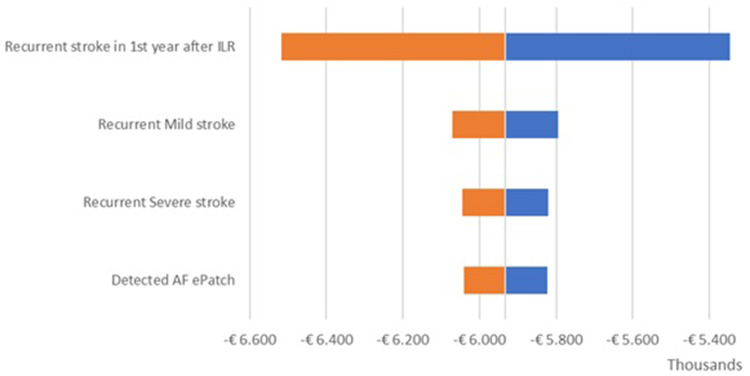
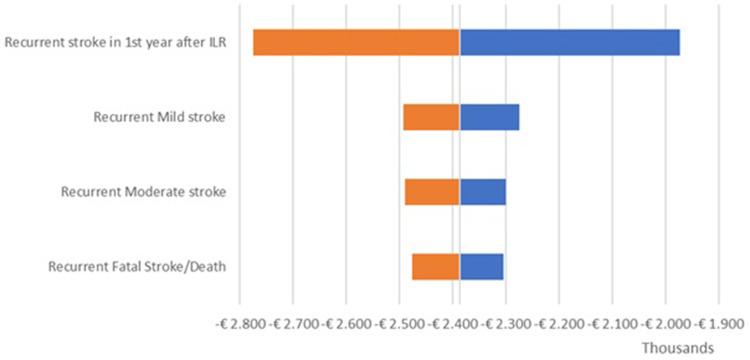
The OWSA results are shown in the tornado diagrams per country (Figure 2 – UK; Figure 3 – Netherlands; Figure 4 – Sweden) which present the variation in output of the univariate sensitivity analysis of the parameters (in order of influence) having the largest single impact on the base case difference in total costs. The key driver of the difference in total costs was the recurrent stroke in the first year after ILR. Variations in other clinical parameters had a minor effect on the base case results.
Figure 2.
Incremental results one-way sensitivity analysis – UK.
Abbreviations: AF, atrial fibrillation; ILR, implantable loop recorder.
Figure 3.
Incremental results one-way sensitivity analysis – Netherlands.
Abbreviations: AF, atrial fibrillation; ILR, implantable loop recorder.
Figure 4.
Incremental results one-way sensitivity analysis – Sweden.
Abbreviations: ILR, implantable loop recorder. Manuscript: Cost-minimization model in Cryptogenic Stroke: ePatch vs Implantable Loop Recorder in Patients from the UK, Netherlands, and Sweden.
Scenario Analyses
Scenario 1
In the context of scenario analyses, the occurrence of recurrent strokes in the first year was contingent upon the monitoring method and detection rates of AF. The only input parameters that were changed are:
Recurrent stroke in 1st year after ePatch = 6.40% (MonDAFIS trial62
Recurrent stroke in 1st year after ILR = 7.10% (CRYSTAL AF trial16
Detected AF ePatch = 5.80% (MonDAFIS trial62
Detected AF ILR = 8.90% (CRYSTAL AF trial (6 months)16
Table 5 provides an overview of the clinical findings concerning the difference between the ePatch and ILR arms.
Table 5.
Scenario 1 Analyses – Clinical Results for a Cohort of 1,000 Patients – Same Treatment Modalities – UK, Sweden and the Netherlands
| ePatch | ILR-only | Difference ePatch – ILR-only | |
|---|---|---|---|
| Number of patients with detected AF in a cohort of 1,000 patients | 58 | 87 | −29 |
| Strokes | |||
| Number of recurrent mild strokes | 26.9 | 29.8 | −2.9 |
| Number of recurrent moderate strokes | 16.6 | 18.5 | −1.8 |
| Number of recurrent severe strokes | 6.4 | 7.1 | −0.7 |
| Number of recurrent fatal strokes/deaths | 14.1 | 15.6 | −1.5 |
| Total number of recurrent strokes | 64.0 | 71.0 | −7.0 |
| Bleedings | |||
| Number of major bleedings | 1.2 | 1.8 | −0.6 |
| Number of CRNM bleed events | 2.4 | 3.6 | −1.2 |
| Total number of bleedings | 3.6 | 5.4 | −1.8 |
| Infections/extrusions | |||
| Number of infections | 0.0 | 7.0 | −7.0 |
| Number of extrusions | 0.0 | 22.5 | −22.5 |
Abbreviations: AF, atrial fibrillation; CRNM, clinically relevant non-major; ILR, implantable loop recorder.
Table 6 provides a breakdown of the total cost differences between the ePatch and ILR arms for all three countries.
Table 6.
Scenario 1 Analyses - Economic Results for a Cohort of 1,000 Patients – Same Treatment Modalities
| UK | Netherlands | Sweden | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ePatch | ILR-only | Difference ePatch – ILR-only | ePatch | ILR-only | Difference ePatch – ILR-only | ePatch | ILR-only | Difference ePatch – ILR-only | |
| Device | €170,000 (£150,000) | €4,153,752 (£3,669,757) | -€3,983,752 (-£3,519,757) |
€ 140,000 | € 7,074,670 | -€ 6,934,670 | €220,000 (kr 2,424,000) |
€2,284,771 (kr 25,521,352) |
-€2,064,771 (-kr 23,097,352) |
| Fatal strokes/Death | €64,816 (£57,263) | €71,905 (£63,527) | -€7,089 (-£6,263) |
€ 46,230 | € 51,286 | -€ 5,056 | €185,002 (kr 2,066,507) |
€185,002 (kr 2,066,507) |
€0 (kr 0) |
| Recurrent strokes | €815,211 (£720,223) | €904,375 (£798,997) | -€89,164 (-£78,774) |
€ 2,382,760 | € 2,643,374 | -€ 260,614 | €727,419 (kr 8,125,413) |
€727,419 (kr 8,125,413) |
€0 (kr 0) |
| Infections | €0 (£0) | €15,846 (£14,000) | -€15,846 (-£14,000) |
€ 0 | € 14,000 | -€ 14,000 | €0 (kr 0) | €2,291 (kr 25,585) |
-€2,291 (-kr 25,585) |
| Bleeding events | €11,854 (£10,473) |
€17,780 (£15,708) |
-€5,926 (-£5,236) |
€ 24,398 | € 36,596 | -€ 12,198 | €3,524 (kr 39,367) |
€8,354 (kr 93,319) |
-€4,830 (-kr 53,952) |
| Aspirin costs | €97,561 (£86,193) | €94,558 (£83,540) | €3,003 (£2,653) |
€ 129,883 | € 125,885 | € 3,998 | €40,552 (kr 452,979) |
€38,435 (kr 429,327) |
€2,117 (kr 23,652) |
| OAC costs | €42,950 (£37,945) | €64,422 (£56,916) | -€21,473 (-£18,971) |
€ 58,891 | € 88,334 | -€ 29,443 | €25,962 (kr 290,002) |
€61,543 (kr 687,450) |
-€35,581 (-kr 397,448) |
| Total costs | €1,202,391 (£1,062,097) | €5,322,639 (£4,702,445) |
-€4,120,248 (-£3,640,348) |
€ 2,782,162 | € 10,034,146 | -€ 7,251,984 |
€1,202,459 (kr 13,398,268) |
€3,307,815 (kr 36,948,954) |
-€2,105,356 (-kr 23,550,686) |
| Cost per patient with detected AF | €20,731 (£18,312) | €61,182 (£54,053) | -€40,451 (-£35,741) |
€ 47,968 | € 115,338 | -€ 67,370 | €32,765 (kr 365,075) |
€38,022 (kr 424,713) |
-€5,257 (-kr 59,637) |
| Cost per 1 patient monitored | €1,202 (£1,062) |
€5,323 (£4,702) |
-€4,120 (-£3,640) |
€ 2,782 | € 10,034 | -€ 7,252 | €1,202 (kr 13,398) | €3,308 (kr 36,949) | -€2,105 (-kr 23,551) |
Note: Percentage of undetected AF patients in ePatch group receive ILR for diagnosis.
Abbreviations: AF, atrial fibrillation; ILR, implantable loop recorder; OAC, oral anticoagulant.
The economic findings of the scenario analyses in all three countries were consistent with the base case results, leading the researchers to conclude that using 14-day ECG monitoring with ePatch for diagnosing AF in cryptogenic stroke patients is a cost-saving alternative to using ILR.
Scenario 2
In the context of scenario analyses, the occurrence of recurrent strokes in the first year was contingent upon the monitoring method and detection rates of AF. The only input parameters that were changed in Scenario 2 are:
Recurrent stroke in 1st year after ePatch = 6.40% (MonDAFIS trial62
Recurrent stroke in 1st year after ILR = 7.10% (CRYSTAL AF trial16
Detected AF ePatch = 9.80% (AF SPICE)65
Detected AF ILR = 10.70% (PER DIEM trial61
Table 7 provides an overview of the clinical findings concerning the difference between the ePatch and ILR arms.
Table 7.
Scenario 2 Analyses – Clinical Results for a Cohort of 1,000 Patients – Same Treatment Modalities – UK, Sweden and the Netherlands
| ePatch | ILR-only | Difference ePatch – ILR-only | |
|---|---|---|---|
| Number of patients with detected AF in a cohort of 1,000 patients | 98 | 105 | −7 |
| Strokes | |||
| Number of recurrent mild strokes | 26.9 | 29.8 | −2.9 |
| Number of recurrent moderate strokes | 16.6 | 18.5 | −1.8 |
| Number of recurrent severe strokes | 6.4 | 7.1 | −0.7 |
| Number of recurrent fatal strokes/deaths | 14.1 | 15.6 | −1.5 |
| Total number of recurrent strokes | 64.0 | 71.0 | −7.0 |
| Bleedings | |||
| Number of major bleedings | 2.0 | 2.1 | −0.1 |
| Number of CRNM bleed events | 4.1 | 4.4 | −0.3 |
| Total number of bleedings | 6.1 | 6.5 | −0.4 |
| Infections/extrusions | |||
| Number of infections | 0.0 | 7.0 | −7.0 |
| Number of extrusions | 0.0 | 22.5 | −22.5 |
Abbreviations: AF, atrial fibrillation; CRNM, clinically relevant non-major; ILR, implantable loop recorder.
Table 8 provides a breakdown of the total cost differences between the ePatch and ILR arms for all three countries.
Table 8.
Scenario 2 Analyses - Economic Results for a Cohort of 1,000 Patients – Same Treatment Modalities
| UK | Netherlands | Sweden | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ePatch | ILR-only | Difference ePatch – ILR-only | ePatch | ILR-only | Difference ePatch – ILR-only | ePatch | ILR-only | Difference ePatch – ILR-only | |
| Device | €170,000 (£150,000) | €4,153,752 (£3,669,757) | -€3,983,752 (-£3,519,757) |
€ 140,000 | € 7,074,670 | -€ 6,934,670 | €170,000 (kr 1,899,000) |
€2,284,771 (kr 25,521,352) |
-€2,114,771 (-kr 23,622,352) |
| Fatal strokes/Death | €64,816 (£57,263) | €71,905 (£63,527) | -€7,089 (-£6,263) |
€ 46,230 | € 51,286 | -€ 5,056 | €251,917 (kr 2,813,967) |
€279,471 (kr 3,121,745) |
-€27,553 (-kr 307,778) |
| Recurrent strokes | €815,211 (£720,223) | €904,375 (£798,997) | -€89,164 (-£78,774) |
€ 2,382,760 | € 2,643,374 | -€ 260,614 | €990,528 (kr 11,064,393) |
€1,098,867 (kr 12,274,561) |
-€108,339 (-kr 1,210,168) |
| Infections | €0 (£0) | €15,846 (£14,000) | -€15,846 (-£14,000) |
€ 0 | € 14,000 | -€ 14,000 | €0 (kr 0) |
€2,291 (kr 25,585) |
-€2,291 (-kr 25,585) |
| Bleeding events | €20,029 (£17,695) |
€21,376 (£18,885) |
-€1,347 (-£1,190) |
€ 41,225 | € 43,998 | -€ 2,773 | €9,411 (kr 105,121) |
€10,044 (kr 112,193) |
-€633 (-kr 7,072) |
| Aspirin costs | €93,418 (£82,533) | €92,735 (£81,930) | €683 (£603) |
€ 124,368 | € 123,459 | € 909 | €37,972 (kr 424,154) |
€37,694 (kr 421,054) |
€278 (kr 3,100) |
| OAC costs | €72,570 (£64,114) | €77,452 (£68,427) | -€4,882 (-£4,313) |
€ 99,506 | € 106,200 | -€ 6,694 | €69,327 (kr 774,391) |
€73,990 (kr 826,485) |
-€4,664 (-kr 52,094) |
| Total costs | €1,236,044 (£1,091,828) | €5,337,442 (£4,715,523) |
-€4,101,398 (-£3,623,695) |
€ 2,834,088 | € 10,056,987 | -€ 7,222,899 |
€1,529,154 (kr 17,081,026) |
€3,787,128 (kr 42,302,974) |
-€2,257,973 (-kr 25,221,948) |
| Cost per patient with detected AF | €12,613 (£11,141) | €51,031 (£45,085) | -€38,418 (-£33,944) |
€ 28,919 | € 96,154 | -€ 67,235 | €15,604 (kr 174,296) |
€36,208 (kr 404,455) |
-€20,605 (-kr 230,159) |
| Cost per 1 patient monitored | €1,236 (£1,092) |
€5,337 (£4,716) |
-€4,101 (-£3,624) |
€ 2,834 | € 10,057 | -€ 7,223 | €1,529 (kr 17,081) | €3,787 (kr 42,303) | -€2,258 (-kr 25,222) |
Note: Percentage of undetected AF patients in ePatch group receive ILR for diagnosis.
Abbreviations: AF, atrial fibrillation; ILR, implantable loop recorder; OAC, oral anticoagulant.
The economic findings of the scenario analyses in all three countries were consistent with the base case results, leading the researchers to conclude that using 14-day ECG monitoring with ePatch for diagnosing AF in cryptogenic stroke patients is a cost-saving alternative to using ILR.
Discussion
Our study indicates that immediate use of ePatch for AF detection after cryptogenic stroke is more advantageous than immediate use of ILR as use of ePatch was associated with significantly lower total cost per patient with detected AF. Furthermore, adopting ePatch as the primary approach for AF diagnosis post-cryptogenic stroke resulted in substantial cost savings across all three countries and all three scenarios compared to using ILR.
Our study presents the results of a distinctive analytical framework that compares the costs of two diagnostic methods from the viewpoint of three major European markets in a cost-minimization context.
There are two key assumptions in this analysis. First, AF detection rates vary depending on the monitoring modality (ePatch vs ILR). Various detection rates from several studies7,16,61,62 were used to assess the impact of AF detection rates on the overall results. Second, the impact of ePatch monitor placement immediately following a stroke versus ILR placement was considered. Several studies7,16,61,62 reported different recurrent stroke rates within the first year, depending on the monitoring modality (ePatch vs ILR). To understand the effects, we explored these recurrent stroke rates by applying different studies across various scenarios, including the base case and two additional scenarios.
Our study’s findings are consistent with those of a retrospective database analysis that compared mid-term monitors to long-term and short-term monitors in the US.67 The authors concluded that given the superior results of the mid-term monitors in terms of both patient care and hospital savings, hospitals are encouraged to enforce protocols that favor the mid-term monitors over the long-term and short-term monitors. Additionally, a Canadian study found that 30-day cardiac rhythm monitoring is likely cost-effective for preventing recurrent strokes in cryptogenic patients, while 14-day monitoring is an attractive value alternative, especially for lower risk patients.68
The literature contains results of cost-effectiveness modeling for ILR; however, these authors employed a lifetime horizon, which sets their approach apart from our study.60,68–70 A few studies have reported the results of cost and healthcare resource use comparisons of mid-term cardiac monitors versus short-term Holter monitoring (that was not in the scope of our analyses) within the European or US context. Kaura et al (2019)71 conducted a budget impact from a UK perspective and concluded that mid-term cardiac monitoring is a cost saving alternative to 24-hour Holter. Additionally, while Chandratheva et al (2017) also found that mid-term cardiac monitoring is cost saving compared to 72-hour Holter, 3-day patch, and in-clinic monitoring,72 Ghosh et al (2018)73 reported that a mid-term cardiac monitor is more costly than a 24-hour Holter monitor. Steinhubl et al (2018) conducted a randomized clinical trial in the US, revealing that mid-term cardiac monitoring increased healthcare resource usage for AF-related therapeutic interventions, but reduced all-cause emergency department visits or inpatient stays.74
Study limitations were partly inherited from the original publication.37 A one-year time horizon overlooks strokes occurring beyond this period and fails to account for the ongoing costs associated with severe strokes that result in disabilities requiring long-term care. Consequently, this limited timeframe underestimates the true costs for the stroke group, as expenses for permanent care extend well beyond the index year. Based on recently published NOAH-AFNET and Artesia studies, the model assumes that ILR detected AF within the first 6 months following the index event may have contributed to the stroke. While the battery life of standard ILR devices (eg Medtronic Reveal LINQ II) can last up to 4.5 years,75 the clinical significance of AF identified distant from the index stroke is unknown. AF detection by the ILR was limited to 6 months to employ a conservative approach to this model. Additionally, all scenarios assumed equal efficacy among antithrombotic agents, while in reality, each drug has a unique mechanism of action, and physicians may recommend one over another under specific circumstances.76 The adopted payer perspective made the analysis conservative as it omitted the societal costs of recurrent strokes resulting from lost productivity, early retirement, and informal care provided by relatives and family members. Another limitation is the use of a mean price of ePatch. The final actual price may be higher or lower depending on various factors, including the specific service model chosen for ePatch, number of patients served or volume committed, and country-specific conditions and regulations. These variables can impact the overall price of ePatch, but would not alter the main conclusions of the model. Due to the inclusion of studies with diverse populations, the clinical conclusions drawn may not accurately reflect real-world scenarios and could either underestimate or overestimate the true clinical outcomes. A limitation of the model is that it does not fully account for variations in monitoring practices across regions or the need for individualized approaches in AF detection. In real-life settings, differences in patient characteristics and the variability in screening methods and durations may affect AF detection outcomes beyond those reflected in standardized guidelines. In addition, variations in demographic characteristics, comorbidities, healthcare settings, and treatment protocols across different study populations can lead to discrepancies in results. Consequently, the generalizability of these findings to broader, more heterogeneous patient groups may be limited, potentially impacting conclusions and their applicability to everyday clinical practice. Given the inclusion of studies with varied populations, the clinical conclusions may not fully represent real-world situations and could either underestimate or overestimate actual clinical outcomes. Differences in demographic characteristics, comorbidities, healthcare settings, and treatment protocols across the study populations can result in inconsistencies in results. As a result, the applicability of these findings to a broader, more diverse patient population might be limited, potentially affecting their relevance to everyday clinical practice. Furthermore, several of the trials analyzed, including FIND-AF, PER DIEM, MonDAFIS, and AF SPICE, involved the randomization of patients with ischemic stroke rather than specifically targeting those with cryptogenic stroke. This broader inclusion criterion may dilute the findings’ relevance to patients with cryptogenic stroke. Additionally, both MonDAFIS and AF SPICE trials included patients with TIA in their study populations. The inclusion of these different patient groups could introduce variability in the results, potentially limiting their applicability to the more specific cohort of patients with cryptogenic stroke. These methodological differences highlight the need for caution when extrapolating the study results to this particular patient population.
The findings of the study resonate with the results of the original analysis and provide the scientific foundation for decision-making processes both at the hospital and policy-making levels. Additionally, these findings may be applicable to other healthcare systems with similar cost constraints and levels of healthcare development, as the model offers a flexible framework that can support AF monitoring strategies in resource-limited settings. Prolonged AF monitoring in cryptogenic stroke patients is suggested by clinical guidelines. Our study results reinforce this clinical approach, recommending extended ambulatory monitoring through the use of an adhesive patch and modern ECG data analysis algorithms.30,36,77
Conclusion
By implementing an initial 14-day ePatch extended wear Holter monitoring strategy for cryptogenic stroke patients, as opposed to proceeding directly to ILR, cost savings per patient with detected AF ranged from 3.4 times in Sweden to 6.0 times in the United Kingdom. The achieved cost savings can be predominantly attributed to the reduced expenses associated with the devices, stemming from lower costs incurred in utilizing ePatch as opposed to ILR. Additionally, a significant factor contributing to the overall savings is the preventive aspect, as the implementation of ePatch not only resulted in cost-effective monitoring, but also led to the avoidance of a greater number of recurrent strokes compared to relying solely on ILR. These findings suggest scalable cost savings for other healthcare systems with similar resource constraints, offering a financially viable approach to enhancing stroke monitoring. However, the one-year time horizon of the model may underestimate the true costs for the stroke group, as it fails to account for strokes occurring beyond this period and the ongoing expenses related to severe strokes requiring long-term care.
The combined effect of these and other factors underscores the economic advantages associated with adopting a diagnostic strategy that incorporates ePatch extended wear Holter, emphasizing its dual impact on reducing device-related expenditures and mitigating the incidence of strokes. The results of this cost-minimization analysis are in-line with the findings of the original research conducted in the US. Our findings reinforce the growing number of clinical recommendations that endorse early prolonged ECG monitoring in cryptogenic stroke patients, which could potentially lead to updates in stroke monitoring guidelines.
Funding Statement
Philips funded all research activities for this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. The authors express gratitude to Sanjay Verma for proofreading and editorial support.
Disclosure
VL, PP, MW, RvL, AD and GM are the employees of Philips. JE has received consultant or lecture fees from Roche Diagnostics, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim, Piotrode and Philips, and research grants from the Swedish Research Council, The Swedish Heart Lung Foundation, The Swedish Innovation Agency, and The Stockholm Region. The authors report no other conflicts of interest in this work.
References
- 1.Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of stroke in Europe: thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke. 2020;51(8):2418–2427. doi: 10.1161/STROKEAHA.120.029606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmis A, Vardas P, Townsend N, et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022;43(8):716–799. doi: 10.1093/eurheartj/ehab892 [DOI] [PubMed] [Google Scholar]
- 3.Unnithan AMDJ, Mehta P. Hemorrhagic Stroke. [Updated 2023 May 8]. In: statPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559173/. Accessed November 28, 2024. [Google Scholar]
- 4.Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. New Engl J Med. 2016;374(16):1533–1542. doi: 10.1056/NEJMoa1412981 [DOI] [PubMed] [Google Scholar]
- 5.Amarenco P, Lavallée PC, Monteiro Tavares L, et al. Five-year risk of stroke after TIA or minor ischemic stroke. New Engl J Med. 2018;378(23):2182–2190. doi: 10.1056/NEJMoa1802712 [DOI] [PubMed] [Google Scholar]
- 6.Andersen SD, Gorst-Rasmussen A, Lip GY, Bach FW, Larsen TB. Recurrent stroke: the value of the CHA2DS2VASc score and the essen stroke risk score in a nationwide stroke cohort. Stroke. 2015;46(9):2491–2497. doi: 10.1161/STROKEAHA.115.009912 [DOI] [PubMed] [Google Scholar]
- 7.Wachter R, Gröschel K, Gelbrich G, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AF(RANDOMISED)): an open-label randomised controlled trial. Lancet Neurol. 2017;16(4):282–290. doi: 10.1016/S1474-4422(17)30002-9 [DOI] [PubMed] [Google Scholar]
- 8.Khanevski AN, Bjerkreim AT, Novotny V, et al. Recurrent ischemic stroke: incidence, predictors, and impact on mortality. Acta neurol Scand. 2019;140(1):3–8. doi: 10.1111/ane.13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao J, Khalid Z, Scallan C, Morillo C, O’Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke. 2007;38(11):2935–2940. doi: 10.1161/STROKEAHA.106.478685 [DOI] [PubMed] [Google Scholar]
- 10.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32(12):2735–2740. doi: 10.1161/hs1201.100209 [DOI] [PubMed] [Google Scholar]
- 11.Andrade JG, Field T, Khairy P. Detection of occult atrial fibrillation in patients with embolic stroke of uncertain source: a work in progress. Front Physiol. 2015;6:100. doi: 10.3389/fphys.2015.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS stroke data bank. Ann. Neurol. 1989;25(4):382–390. doi: 10.1002/ana.410250410 [DOI] [PubMed] [Google Scholar]
- 13.Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30(12):2513–2516. doi: 10.1161/01.STR.30.12.2513 [DOI] [PubMed] [Google Scholar]
- 14.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429–438. doi: 10.1016/S1474-4422(13)70310-7 [DOI] [PubMed] [Google Scholar]
- 15.Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14(9):903–913. doi: 10.1016/S1474-4422(15)00132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. New Engl J Med. 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 17.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. New Engl J Med. 2012;366(2):120–129. doi: 10.1056/NEJMoa1105575 [DOI] [PubMed] [Google Scholar]
- 18.Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke. 2014;45(2):520–526. doi: 10.1161/STROKEAHA.113.003433 [DOI] [PubMed] [Google Scholar]
- 19.Verma N, Ziegler PD, Liu S, Passman RS. Incidence of atrial fibrillation among patients with an embolic stroke of undetermined source: insights from insertable cardiac monitors. Int J Stroke. 2019;14(2):146–153. doi: 10.1177/1747493018798554 [DOI] [PubMed] [Google Scholar]
- 20.Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013;44(11):3103–3108. doi: 10.1161/STROKEAHA.113.002329 [DOI] [PubMed] [Google Scholar]
- 21.Friberg L, Rosenqvist M, Lindgren A, Terént A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45(9):2599–2605. doi: 10.1161/STROKEAHA.114.006070 [DOI] [PubMed] [Google Scholar]
- 22.Asberg S, Henriksson KM, Farahmand B, et al. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14,529 patients in the Swedish stroke register. Stroke. 2010;41(7):1338–1342. doi: 10.1161/STROKEAHA.110.580209 [DOI] [PubMed] [Google Scholar]
- 23.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Archives of Internal Medicine. 1987;147(9):1561–1564. doi: 10.1001/archinte.1987.00370090041008 [DOI] [PubMed] [Google Scholar]
- 24.Thygesen SK, Frost L, Eagle KA, Johnsen SP. Atrial fibrillation in patients with ischemic stroke: a population-based study. Clin Epidemiol. 2009;1:55–65. doi: 10.2147/CLEP.S4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannon N, Sheehan O, Kelly L, et al. Stroke associated with atrial fibrillation--incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis. 2010;29(1):43–49. doi: 10.1159/000255973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Internal Med. 2007;147(8):590–592. doi: 10.7326/0003-4819-147-8-200710160-00018 [DOI] [PubMed] [Google Scholar]
- 27.(European Atrial Fibrillation Trial) Study Group E, EAFT. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet (London, England). 1993;342(8882):1255–1262. doi: 10.1016/0140-6736(93)92358-Z [DOI] [PubMed] [Google Scholar]
- 28.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. New Engl J Med. 2011;364(9):806–817. doi: 10.1056/NEJMoa1007432 [DOI] [PubMed] [Google Scholar]
- 29.McIntyre WF, Benz AP, Becher N, et al. Direct oral anticoagulants for stroke prevention in patients with device-detected atrial fibrillation: a study-level meta-analysis of the NOAH-AFNET 6 and ARTESiA trials. Circulation. 2024;149(13):981–988. doi: 10.1161/CIRCULATIONAHA.123.067512 [DOI] [PubMed] [Google Scholar]
- 30.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. 2021;52(7):e364–e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 31.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 32.Choe WC, Passman RS, Brachmann J, et al. A comparison of atrial fibrillation monitoring strategies after cryptogenic stroke (from the cryptogenic stroke and underlying AF trial). Am j Cardiol. 2015;116(6):889–893. doi: 10.1016/j.amjcard.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 33.Ziegler PD, Rogers JD, Ferreira SW, et al. Long-term detection of atrial fibrillation with insertable cardiac monitors in a real-world cryptogenic stroke population. Int J Cardiol. 2017;244:175–179. doi: 10.1016/j.ijcard.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 34.Galli A, Ambrosini F, Lombardi F. Holter monitoring and loop recorders: from research to clinical practice. Arrhythm Electrophysiol Rev. 2016;5(2):136–143. doi: 10.15420/AER.2016.17.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sposato LA, Cipriano LE, Saposnik G, Ruiz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377–387. doi: 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 36.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 37.Medic G, Kotsopoulos N, Connolly MP, et al. Mobile cardiac outpatient telemetry patch vs implantable loop recorder in cryptogenic stroke patients in the US - cost-minimization model. Med Devices. 2021;14:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NICE. Implantable cardiac monitors to detect atrial fibrillation after cryptogenic stroke. Available from: www.nice.org.uk/guidance/dg41. Accessed December 13, 2023. 2020.
- 39.NHS. Stroke nurses first in the UK to implant cardiac monitors in patients. Available from: https://www.uclh.nhs.uk/news/stroke-nurses-first-uk-implant-cardiac-monitors-patients. Accessed November 7, 2024. 2022.
- 40.FMS. Cardiale emboliebron herseninfarct. Project nr: 843004113. Einddatum: 12-2021. Available from: https://richtlijnendatabase.nl/richtlijn/herseninfarct_en_hersenbloeding/diagnostiek_bij_herseninfarct_-bloeding/cardiale_emboliebron_herseninfarct.html. Accessed 7th, November 2024. 2021.
- 41.Straat K, Isaksson E, Laska AC, et al. Large variations in atrial fibrillation screening practice after ischemic stroke and transient ischemic attack in Sweden: a survey study. BMC Neurol. 2024;24(1):120. doi: 10.1186/s12883-024-03622-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Socialstyrelsen. [Nationella riktlinjer för vård vid stroke - Stöd för styrning och ledning] National guidelines for stroke care - Support for governance and management. ISBN 978-91-7555-509-6. www.socialstyrelsen.se 2020.
- 43.Kanters TA, Bouwmans CAM, van der Linden N, Tan SS, Hakkaart-van Roijen L. Update of the Dutch manual for costing studies in health care. PLoS One. 2017;12(11):e0187477. doi: 10.1371/journal.pone.0187477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NICE. National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword. Accessed December 13, 2023. 2013. [PubMed]
- 45.TLV. The Dental and Pharmaceutical Benefits Agency (TLV) General guidelines for economic evaluations from the pharmaceutical benefits board. LFNAR. 2003;2003(2):2003. [Google Scholar]
- 46.CPI SCB, Fixed Index Numbers (1980=100). Statistiska Centralbyrån. Available from: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/prices-and-consumption/consumer-price-index/consumer-price-index-cpi/pong/tables-and-graphs/consumer-price-index-cpi/cpi-fixed-index-numbers-1980100/. Accessed December 13, 2023. 2023.
- 47.ONS. Office for National Statistics. Consumer Price Inflation Index for Medical Services (DKC3). Available from::https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/dkc3/mm23. Accessed December 13, 2023]. 2021.
- 48.CBS. Annual change consumer price index; from 1963. Centraal Bureau voor de Statistiek (CBS). 2022. Available from: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/70936ned/table?fromstatweb. Accessed November 28, 2024.
- 49.DoHSC. Department of Health and Social Care. NHS Reference Costs 2020 to 2021. 2022. Available from: https://www.england.nhs.uk/wp-content/uploads/2022/07/2_National_schedule_of_NHS_costs_FY20-21.xlsx. Accessed December 13, 2023.
- 50.NICE. Reveal LINQ insertable cardiac monitor to detect atrial fibrillation after cryptogenic stroke. Available from: https://allcatsrgrey.org.uk/wp/download/governance/clinical_governance/reveal-linq-insertable-cardiac-monitor-to-detect-atrial-fibrillation-after-cryptogenic-stroke-pdf-2285963451125701.pdf. Accessed December 13, 2023. 2018.
- 51.SS. Viktlistor för NordDRG. Socialstyrelsen. Available from:https://www.socialstyrelsen.se/statistik-och-data/klassifikationer-och-koder/drg/viktlistor/. (2022). Accessed December 13, 2023. 2022.
- 52.CZ. Tarieven Medisch Specialistische Zorg per 1 januari 2022 [Medical Specialist Care Rates as of January 1, 2022]. Available from: https://www.cz.nl/-/media/actueel/voorwaarden/gemiddeld-ongewogen-gecontracteerde-tarieven-msz.pdf?revid=c01bd1eb-8f58-484c-a1ad-17b384e1e0dd. Accessed December 14, 2023. 2022.
- 53.Luengo-Fernandez R, Yiin GS, Gray AM, Rothwell PM. Population-based study of acute- and long-term care costs after stroke in patients with AF. Int J Stroke. 2013;8(5):308–314. doi: 10.1111/j.1747-4949.2012.00812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevanović J, Pompen M, Le HH, Rozenbaum MH, Tieleman RG, Postma MJ. Economic evaluation of apixaban for the prevention of stroke in non-valvular atrial fibrillation in the Netherlands. PLoS One. 2014;9(8):e103974. doi: 10.1371/journal.pone.0103974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lekander I, Willers C, von Euler M, et al. Relationship between functional disability and costs one and two years post stroke. PLoS One. 2017;12(4):e0174861. doi: 10.1371/journal.pone.0174861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.BNF. Joint Formulary Committee. British National Formulary (Online) www.medicinescomplete.com. 2022. London: BMJ Group and Pharmaceutical Press; 2022. [Google Scholar]
- 57.TLV. Sök priser och beslut i databasen - Tandvårds- och läkemedelsförmånsverket TLV [Search prices and decisions in the database - Dental and pharmaceutical benefits agency TLV]. Available from: https://www.tlv.se/beslut/sok-priser-och-beslut-i-databasen.html.Accessed December 14, 2023. 2023.
- 58.ZiNL. Zorginstituut Nederland (ZiNL). Dutch Drug reimbursement. The Hague, the Netherlands. Available from: https://www.medicijnkosten.nl/.2023. Accessed November 28, 2024.
- 59.Lanitis T, Kongnakorn T, Jacobson L, De Geer A. Cost-effectiveness of apixaban versus warfarin and aspirin in Sweden for stroke prevention in patients with atrial fibrillation. Thrombosis Res. 2014;134(2):278–287. doi: 10.1016/j.thromres.2014.05.027 [DOI] [PubMed] [Google Scholar]
- 60.Sawyer LM, Witte KK, Reynolds MR, et al. Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. J Comparative Effectiveness Res. 2021;10(2):127–141. doi: 10.2217/cer-2020-0224 [DOI] [PubMed] [Google Scholar]
- 61.Buck BH, Hill MD, Quinn FR, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA. 2021;325(21):2160–2168. doi: 10.1001/jama.2021.6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haeusler KG, Kirchhof P, Kunze C, et al. Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): a randomised, open-label, multicentre study. Lancet Neurol. 2021;20(6):426–436. doi: 10.1016/S1474-4422(21)00067-3 [DOI] [PubMed] [Google Scholar]
- 63.Afzal MR, Casmer A, Buck B, et al. Incidence and Risk Factors for Early Explantation of Subcutaneous Cardiac Rhythm Monitors. JACC Clin Electrophysiol. 2020;6(14):1858–1860 [DOI] [PubMed] [Google Scholar]
- 64.Li A, Carlson JJ, Kuderer NM, et al. Cost-effectiveness analysis of low-dose direct oral anticoagulant (DOAC) for the prevention of cancer-associated thrombosis in the United States. Cancer. 2020;126(8):1736–1748 [DOI] [PubMed] [Google Scholar]
- 65.Straat K, Isaksson E, Svennberg E, Wester P, Engdahl J. Study design and preliminary results from the Arterial Fibrillation Screening Post Ischemic Cerebrovascular Events (AF SPICE) study. Poster ESOC. 2024;2024. [Google Scholar]
- 66.Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Economics. 1994;3(2):95–104. doi: 10.1002/hec.4730030206 [DOI] [PubMed] [Google Scholar]
- 67.Tsang JP, Mohan S. Benefits of monitoring patients with mobile cardiac telemetry (MCT) compared with the Event or Holter monitors. Med Devices. 2013;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maervoet J, Bossers N, Borge RP Jr, Hilpert ST, van Engen A, Smala A. Use of insertable cardiac monitors for the detection of atrial fibrillation in patients with cryptogenic stroke in the United States is cost-effective. J Med Economics. 2019;22(11):1221–1234. doi: 10.1080/13696998.2019.1663355 [DOI] [PubMed] [Google Scholar]
- 69.Chew DS, Rennert-May E, Quinn FR, et al. Economic evaluation of extended electrocardiogram monitoring for atrial fibrillation in patients with cryptogenic stroke. Int J Stroke. 2020;16:1747493020974561. [DOI] [PubMed] [Google Scholar]
- 70.Diamantopoulos A, Sawyer LM, Lip GY, et al. Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke. 2016;11(3):302–312. doi: 10.1177/1747493015620803 [DOI] [PubMed] [Google Scholar]
- 71.Kaura A, Sztriha L, Chan FK, et al. Early prolonged ambulatory cardiac monitoring in stroke (EPACS): an open-label randomised controlled trial. Eur J Med Res. 2019;24(1):25. doi: 10.1186/s40001-019-0383-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandratheva A, Pleming J, Dados A. New versus conventional non invasive cardiac monitoring for atrial fibrillation detection in TIA Clinic. Eur Stroke J. 2017;2:117. [Google Scholar]
- 73.Ghosh M, Lochrie N, Mahmood S, Lawrence E, Karen Kee Y. Improving detection of atrial fibrillation after transient ischaemic attack and stroke. Eur Stroke J. 2018;3:228. [Google Scholar]
- 74.Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320(2):146–155. doi: 10.1001/jama.2018.8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medtronic. LINQ II - Insertable Cardiac Monitor - Specifications sheet. Available from: https://asiapac.medtronic.com/content/dam/medtronic-com/us-en/hcp/diagnostics/documents/reveal-linq/linqii-specs.pdf?bypassIM=true. Accessed 13th, August 2024. 2020.
- 76.Kapil N, Datta YH, Alakbarova N, et al. Antiplatelet and anticoagulant therapies for prevention of ischemic stroke. Clin Appl Thrombosis/Hemostasis. 2017;23(4):301–318. doi: 10.1177/1076029616660762 [DOI] [PubMed] [Google Scholar]
- 77.January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130(23):2071–2104. doi: 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]