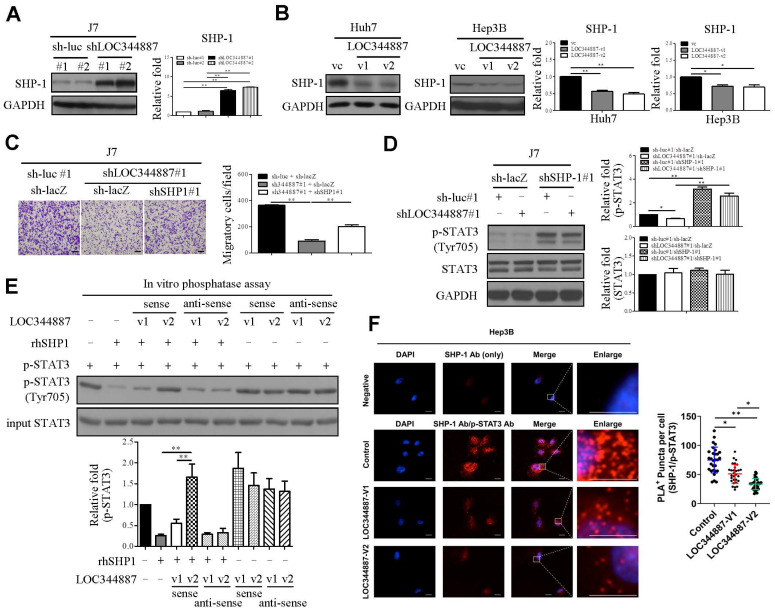
Figure 5.
LOC344887 mediates STAT3 phosphorylation by regulating SHP-1 expression and its interaction with STAT3. SHP-1 immunoblotting was performed to assess SHP-1 expression in (A) LOC344887 stable knockdown J7 cell lines and (B) LOC344887-v1/-v2 stable overexpression Huh7 and Hep3B cell lines, showing significantly enhanced and reduced expression of SHP-1, respectively. Quantitative results of immunoblots analysis (n = 3) were presented relative to the control groups using GAPDH as loading control. (C) Effects of LOC344887 and SHP-1 expression on cell migration were assessed by knocking down LOC344887 and/or SHP-1 in J7 cells transduced with shLOC344887#1 and/or SHP-1 (shSHP-1#1), showing knockdown of SHP-1 significantly reverted the reduced cell motility by shLOC344887. Sh-luc#1 and sh-lacZ served as control groups. The scale bar is 100 μm. (D) The expression levels of p-STAT3 in LOC344887 and/or SHP-1 in knockdown J7 cells were determined to evaluate the combined effects of LOC344887 and SHP-1 on STAT3 phosphorylation at Tyr705. Quantitative results of immunoblots (n = 3) were obtained by normalizing p-STAT3 or STAT3 to the control groups (lane 1: sh-luc#1/sh-lacZ) as GAPDH served as an internal control. (E) In vitro phosphatase assays using recombinant human SHP1 (rhSHP1) incubated in the presence or absence of biotinylated LOC344887 RNA variants (v1 and v2) to allow investigation for the roles of LOC344887-v1/-v2 and SHP1 in mediating STAT3 phosphorylation. The results indicated differential influences from LOC344887-v1 and -v2 sense strands (lane 3, 4) on preventing dephosphorylation effects from rhSHP1. The quantitative results (n = 3) were presented relative to the control group (lane 1). (F) The differential influences of LOC344887-v1 and -v2 on the interaction between SHP-1 and p-STAT3 (Tyr705) was assessed via Duolink proximity ligation assay (PLA) using control-, LOC344887-v1- or LOC344887-v2-overexpressing J7 cells. The results showed a significantly lower association between SHP1/p-STAT3 and LOC344887-v2-overexpression, suggesting the lowest dephosphorylation rate takes place. The proximity of SHP1/p-STAT3 was defined as less than 40 nm and visualized by discrete red fluorescent puncta under fluorescence microscopy. Nuclei were counterstained with DAPI. Scale bars: 10 μm. *, P < 0.05; **, P < 0.01.