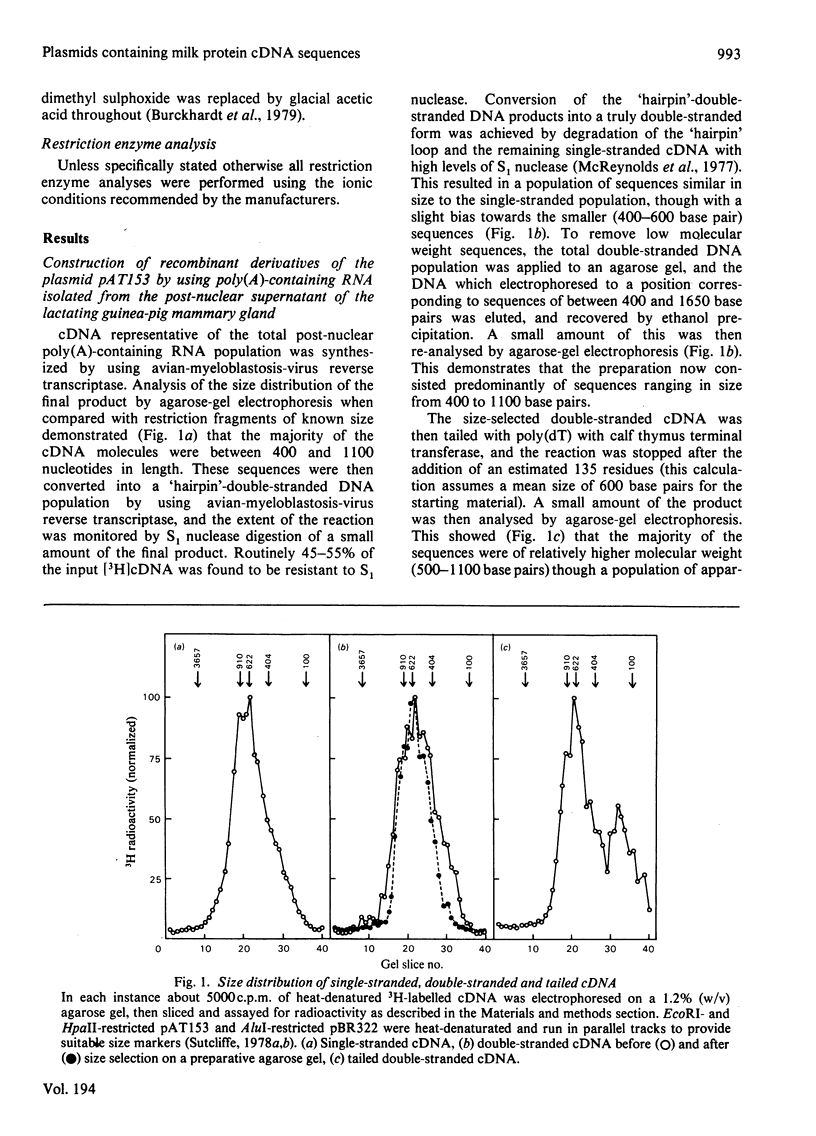
Abstract
A complementary DNA (cDNA) plasmid library has been constructed in the plasmid pAT153, using poly(A)-containing RNA isolated from the lactating guinea-pig mammary gland as the starting material. Double stranded cDNA was inserted into the EcoRI site of the plasmid using poly(dA . dT) tails, then transformed into Escherichia coli HB101. From the resulting colonies we have selected and partially characterized plasmids containing cDNA copies of the mRNAs for casein A, casein B, casein C and alpha-lactalbumin. However, the proportion containing casein C cDNA was exceptionally low, and these contained at best 60% of the mRNA sequence.
Full text
PDF



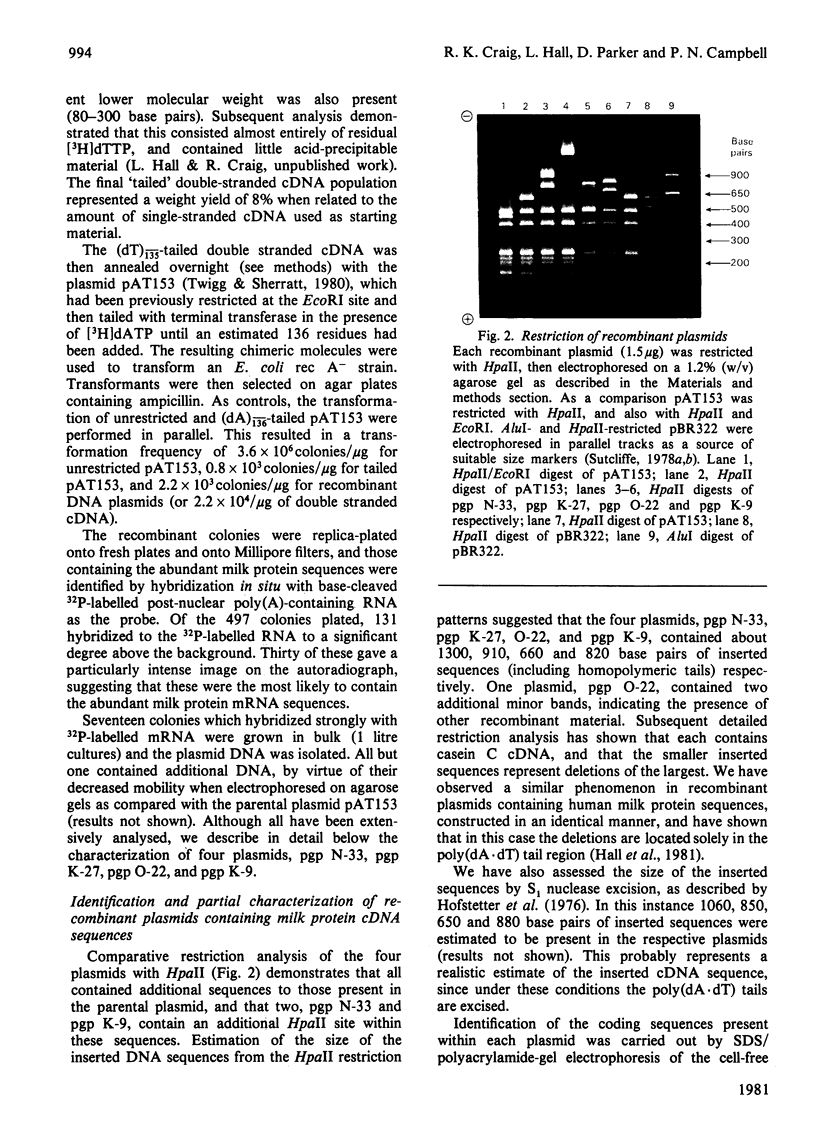
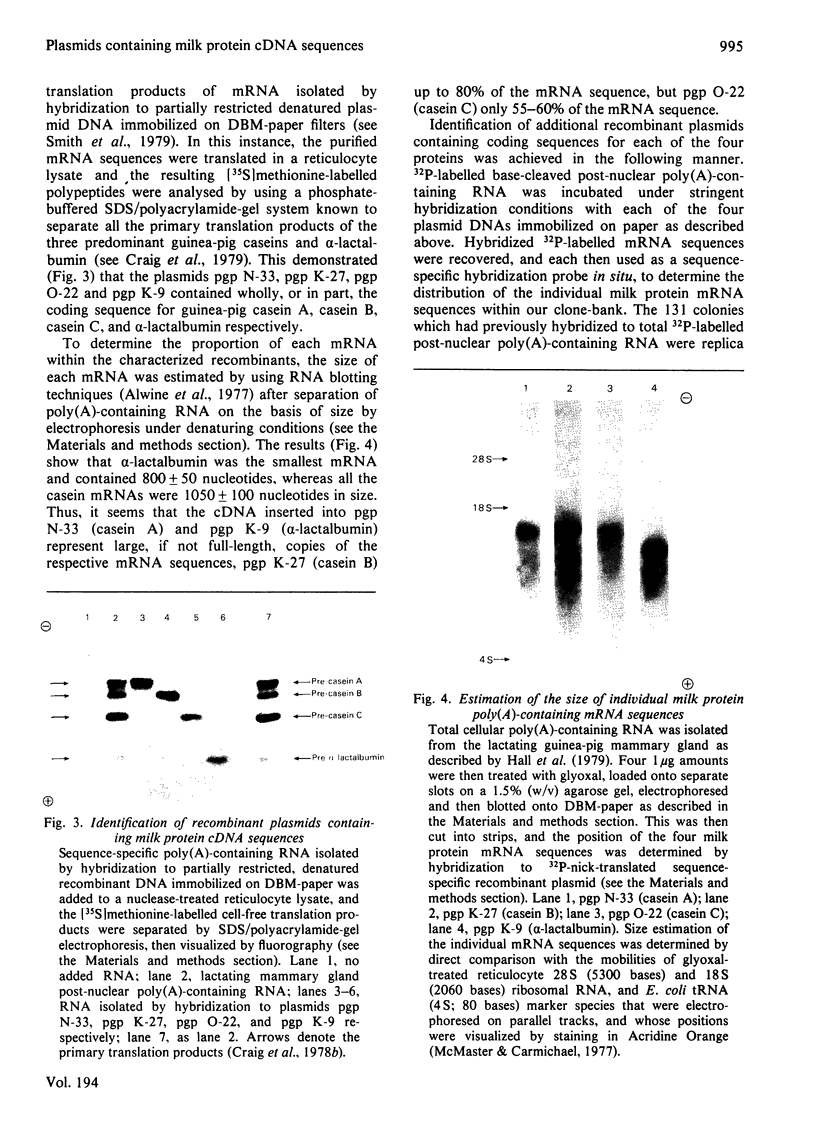
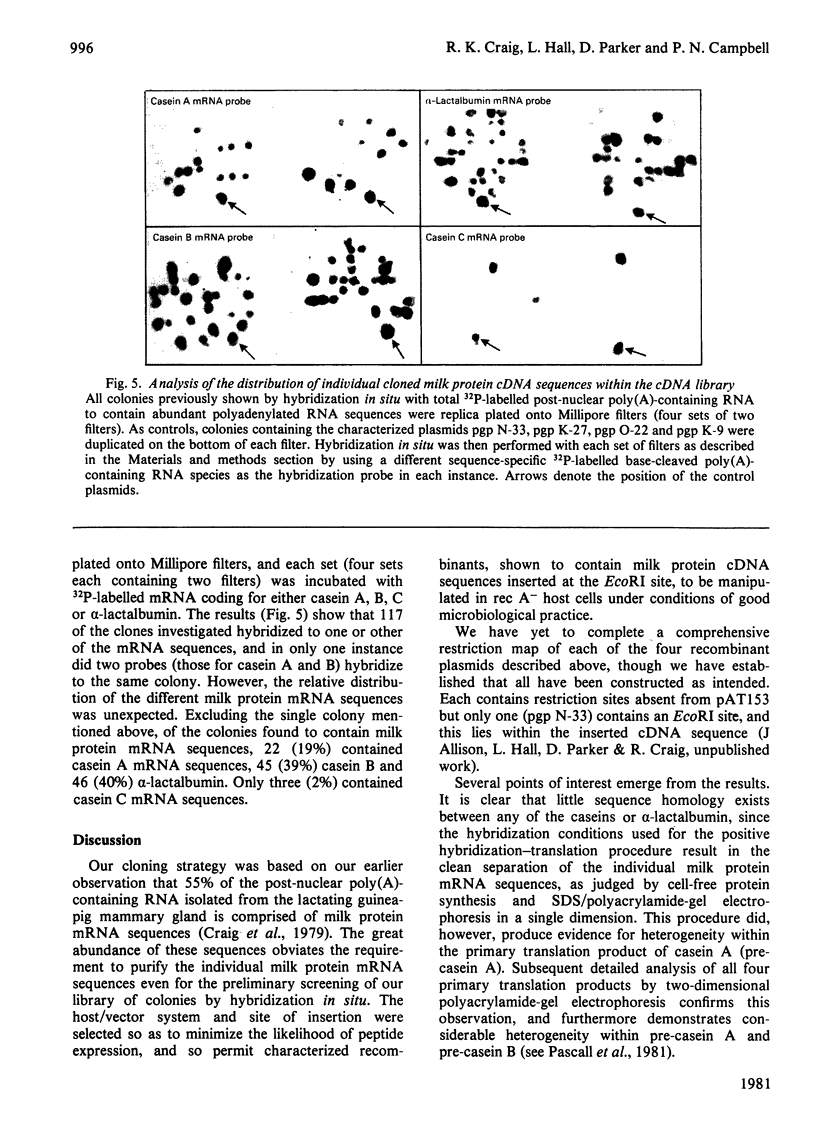


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M. R. Responses of mammary cells to hormones. Int Rev Cytol. 1976;47:1–97. doi: 10.1016/s0074-7696(08)60086-8. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Craig R. K., Herries D. G., Campbell P. N. Differential distribution of poly(A)-containing RNA sequences between the nucleus and post-nuclear supernatant of the lactating guinea-pig mammary gland. Eur J Biochem. 1980 Aug;109(1):183–191. doi: 10.1111/j.1432-1033.1980.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Craig R. K., Herries D. G., Campbell P. N. Relative distribution of post-nuclear poly(A)-containing RNA abundance groups within the nuclear and post-nuclear polyadenylated and non-polyadenylated RNA populations of the lactating guinea-pig mammary gland. Biochem J. 1980 Nov 15;192(2):489–498. doi: 10.1042/bj1920489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Burckhardt J., Telford J., Birnstiel M. L. Detection of labelled RNA species by contact hybridization. Nucleic Acids Res. 1979 Jul 11;6(9):2963–2971. doi: 10.1093/nar/6.9.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burditt L. J., Parker D., Craig R. K., Getova T., Campbell P. N. Differential expression of alpha-lactalbumin and casein genes during the onset of lactation in the guinea-pig mammary gland. Biochem J. 1981 Mar 15;194(3):999–1006. doi: 10.1042/bj1940999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Bathurst I. C., Herries D. G. Post-transcriptional regulation of gene expression in guinea pig tissues. Nature. 1980 Dec 11;288(5791):618–619. doi: 10.1038/288618a0. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Boulton A. P., Harrison O. S., Parker D., Campbell P. N. Studies on the intracellular segregation of polyribosome-associated messenger ribonucleic acid species in the lactating guinea-pig mammary gland. Biochem J. 1979 Sep 1;181(3):737–756. doi: 10.1042/bj1810737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Campbell P. N. mRNA species directing synthesis of milk proteins in normal and tumour tissue from human mammary gland. Nature. 1979 Jan 4;277(5691):54–56. doi: 10.1038/277054a0. [DOI] [PubMed] [Google Scholar]
- Hall L., Davies M. S., Craig R. K. The construction, identification and characterisation of plasmids containing human alpha-lactalbumin cDNA sequences. Nucleic Acids Res. 1981 Jan 10;9(1):65–84. doi: 10.1093/nar/9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter H., Schamböck A., Van Den Berg J., Weissmann C. Specific excision of the inserted DNA segment from hybrid plasmids constructed by the poly(dA). poly (dT) method. Biochim Biophys Acta. 1976 Dec 13;454(3):587–591. doi: 10.1016/0005-2787(76)90286-0. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R. M., Harris R., Patient R. K., Williams J. G. Molecular cloning of cDNA sequences coding for the major alpha- and beta-globin polypeptides of adult Xenopus laevis. Nucleic Acids Res. 1980 Jun 25;8(12):2691–2707. doi: 10.1093/nar/8.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shine J., Seeburg P. H., Martial J. A., Baxter J. D., Goodman H. M. Construction and analysis of recombinant DNA for human chorionic somatomammotropin. Nature. 1977 Dec 8;270(5637):494–499. doi: 10.1038/270494a0. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Searle P. F., Williams J. G. Characterisation of bacterial clones containing DNA sequences derived from Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1979 Feb;6(2):487–506. doi: 10.1093/nar/6.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]