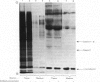
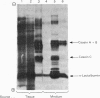
Abstract
1. The expression of alpha-lactalbumin and casein genes was examined in guinea-pig mammary tissue taken from animals both pre- and post-partum. 2. Analysis of total RNA by RNA excess hybridization with sequence-specific complementary DNA probes demonstrated that alpha-lactalbumin mRNA was present late in pregnancy, and that maximum concentrations were present at parturition. Casein gene transcripts were absent late in pregnancy (62 days), but by parturition were present at concentrations identical to those found at all time points examined throughout lactation. 3. Studies using mammary explants in organ culture showed that tissue from pregnant animals, or animals at parturition, synthesized and secreted only alpha-lactalbumin. After parturition, at the onset of casein synthesis, differential rates of secretion of alpha-lactalbumin and the caseins were observed. 4. The results are discussed in terms of the multiple intracellular mechanisms involved in the regulation of milk protein gene expression in the guinea-pig mammary gland.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assairi L., Delouis C., Gaye P., Houdebine L. M., Bousquet M. O., Denamur R. Inhibition by progesterone of the lactogenic effect of prolactin in the pseudopregnant rabbit. Biochem J. 1974 Nov;144(2):245–252. doi: 10.1042/bj1440245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M. R. Responses of mammary cells to hormones. Int Rev Cytol. 1976;47:1–97. doi: 10.1016/s0074-7696(08)60086-8. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Craig R. K., Herries D. G., Campbell P. N. Differential distribution of poly(A)-containing RNA sequences between the nucleus and post-nuclear supernatant of the lactating guinea-pig mammary gland. Eur J Biochem. 1980 Aug;109(1):183–191. doi: 10.1111/j.1432-1033.1980.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Craig R. K., Herries D. G., Campbell P. N. Relative distribution of post-nuclear poly(A)-containing RNA abundance groups within the nuclear and post-nuclear polyadenylated and non-polyadenylated RNA populations of the lactating guinea-pig mammary gland. Biochem J. 1980 Nov 15;192(2):489–498. doi: 10.1042/bj1920489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Hill R. L. Lactose biosynthesis. Rev Physiol Biochem Pharmacol. 1975;72:105–158. doi: 10.1007/BFb0031548. [DOI] [PubMed] [Google Scholar]
- Brew K. The complete amino-acid sequence of guinea-pig -lactalbumin. Eur J Biochem. 1972 May 23;27(2):341–353. doi: 10.1111/j.1432-1033.1972.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Colman A., Lane C. D., Craig R., Boulton A., Mohun T., Morser J. The influence of topology and glycosylation on the fate of heterologous secretory proteins made in Xenopus oocytes. Eur J Biochem. 1981 Jan;113(2):339–348. doi: 10.1111/j.1432-1033.1981.tb05072.x. [DOI] [PubMed] [Google Scholar]
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Bathurst I. C., Herries D. G. Post-transcriptional regulation of gene expression in guinea pig tissues. Nature. 1980 Dec 11;288(5791):618–619. doi: 10.1038/288618a0. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Boulton A. P., Harrison O. S., Parker D., Campbell P. N. Studies on the intracellular segregation of polyribosome-associated messenger ribonucleic acid species in the lactating guinea-pig mammary gland. Biochem J. 1979 Sep 1;181(3):737–756. doi: 10.1042/bj1810737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Parker D., Campbell P. N. The construction, identification and partial characterization of plasmids containing guinea-pig milk protein complementary DNA sequences. Biochem J. 1981 Mar 15;194(3):989–998. doi: 10.1042/bj1940989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Perera P. A., Mellor A., Smith A. E. Initiation and processing in vitro of the primary translation products of guinea-pig caseins. Biochem J. 1979 Nov 15;184(2):261–267. doi: 10.1042/bj1840261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Ganguly R., Mehta N. M., Ganguly N., Banerjee M. R. Glucocorticoid modulation of casein gene transcription in mouse mammary gland. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6466–6470. doi: 10.1073/pnas.76.12.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Campbell P. N. mRNA species directing synthesis of milk proteins in normal and tumour tissue from human mammary gland. Nature. 1979 Jan 4;277(5691):54–56. doi: 10.1038/277054a0. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M., Gaye P. Regulation of casein synthesis in the rabbit mammary gland. Titration of mRNA activity for casein under prolactin and progesterone treatments. Mol Cell Endocrinol. 1975 Jul;3(1):37–55. doi: 10.1016/0303-7207(75)90030-1. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T., Chan K. M., Feigelson P. Translational control of hepatic alpha2u globulin synthesis by growth hormone. Cell. 1978 Nov;15(3):743–750. doi: 10.1016/0092-8674(78)90260-x. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Colman A., Mohun T., Morser J., Champion J., Kourides I., Craig R., Higgins S., James T. C., Applebaum S. W. The Xenopus oocyte as a surrogate secretory system. The specificity of protein export. Eur J Biochem. 1980 Oct;111(1):225–235. doi: 10.1111/j.1432-1033.1980.tb06097.x. [DOI] [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- NELSON W. L., HEYTLER P. G., CIACCIO E. I. Guinea pig mammary gland growth changes in weight, nitrogen and nucleic acids. Proc Soc Exp Biol Med. 1962 Feb;109:373–375. doi: 10.3181/00379727-109-27207. [DOI] [PubMed] [Google Scholar]
- Nakhasi H. L., Quasba P. K. Quantitation of milk proteins and their mRNAs in rat mammary gland at various stages of gestation and lactation. J Biol Chem. 1979 Jul 10;254(13):6016–6025. [PubMed] [Google Scholar]
- Nardacci N. J., Lee J. W., McGuire W. L. Differential regulation of alpha-lactalbumin and casein messenger RNA's in mammary tissue. Cancer Res. 1978 Sep;38(9):2694–2699. [PubMed] [Google Scholar]
- Ono M., Oka T. The differential actions of cortisol on the accumulation of alpha-lactalbumin and casein in midpregnant mouse mammary gland in culture. Cell. 1980 Feb;19(2):473–480. doi: 10.1016/0092-8674(80)90522-x. [DOI] [PubMed] [Google Scholar]
- Ono M., Oka T. alpha-Lactalbumin-casein induction in virgin mouse mammary explants: dose-dependent differential action of cortisol. Science. 1980 Mar 21;207(4437):1367–1369. doi: 10.1126/science.6986657. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., O'Neal D. L., McHugh J. E., Comstock J. P. Progesterone-mediated inhibition of casein mRNA and polysomal casein synthesis in the rat mammary gland during pregnancy. Biochemistry. 1978 Jan 24;17(2):290–297. doi: 10.1021/bi00595a016. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Shuster R. C., Houdebine L. M., Gaye P. Studies on the synthesis of casein messenger RNA during pregnancy in the rabbit. Eur J Biochem. 1976 Dec;71(1):193–199. doi: 10.1111/j.1432-1033.1976.tb11106.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. G., Washburn L. L., Mukherjee A. S., Banerjee M. R. Hormonal regulation of lobulo-alveolar growth, functional differentiation and regression of whole mouse mammary gland in organ culture. J Endocrinol. 1975 Apr;65(1):1–6. doi: 10.1677/joe.0.0650001. [DOI] [PubMed] [Google Scholar]