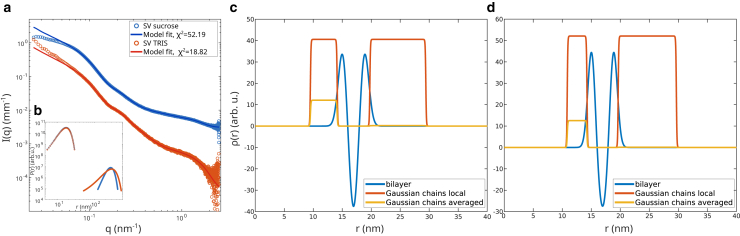
Figure 3.
(a) Azimuthally averaged scattering intensity of the different SV preparations at a detector distance of 3 m and least-square fits. SVs in sucrose buffer were measured at a vesicle concentration of 240 nM, SVs in TRIS buffer were measured at a concentration of 60 nM. The first 30 points of the measured intensity were not included in the fitting process. For the SVs in sucrose buffer, the SAXS curve measured for pure water capillary was subtracted as a background. (b) Bimodal Gaussian size distribution obtained from the fits in (a). The bimodal distribution accounts for the size of the actual SVs (smaller radii) as well as contamination by larger membranous particles (larger radii). The size of the actual vesicles was kept constant during the fitting process, while the size of the larger particles was freely variable. (c) Excess electron density profile of the bilayer described by three Gaussians as well as the proteins described by Gaussian chains for SVs in sucrose buffer obtained from the fits in (a). The excess electron density of the proteins can be described by the typical local excess electron density of the protein patches (Gaussian chains local) or as the spherically averaged contribution (Gaussian chains averaged), which correspond to the excess electron density if the proteins were described by a spherical shell. (d) Excess electron density profiles for SVs in TRIS buffer.