Table 1. Optimization of coupling of amide 1a and silyl enol ether 2aa,b.
| ||||
|---|---|---|---|---|
| Entry | Solvent | Photocatalyst | Conc. [M] | Assay yield (%) |
| 1 | DMF | fac-Ir(ppy)3 | 0.1 | 40 |
| 2 | DMSO | fac-Ir(ppy)3 | 0.1 | 68 |
| 3 | CH3CN | fac-Ir(ppy)3 | 0.1 | 27 |
| 4 | DCM | fac-Ir(ppy)3 | 0.1 | 0 |
| 5 | DCE | fac-Ir(ppy)3 | 0.1 | 12 |
| 6 | THF | fac-Ir(ppy)3 | 0.1 | 18 |
| 7 | PhCl | fac-Ir(ppy)3 | 0.1 | 20 |
| 8 | PhCF3 | fac-Ir(ppy)3 | 0.1 | Trace |
| 9 | EtOH | fac-Ir(ppy)3 | 0.1 | 0 |
| 10 | DMSO | 4CzIPN | 0.1 | 13 |
| 11 | DMSO | [Ir(dtbbpy)(ppy)2][PF6] | 0.1 | 31 |
| 12 | DMSO | [Ir(ppy)2(bpy)]PF6 | 0.1 | 26 |
| 13 | DMSO | Eosin Y | 0.1 | 18 |
| 14c | DMSO | fac-Ir(ppy)3 | 0.1 | 94 (90)d |
| 15c | DMSO | fac-Ir(ppy)3 | 0.2 | 86 |
| 16c | DMSO | fac-Ir(ppy)3 | 0.05 | 77 |
| 17c | DMSO | — | 0.1 | 0 |
| 18e | DMSO | fac-Ir(ppy)3 | 0.1 | 0 |
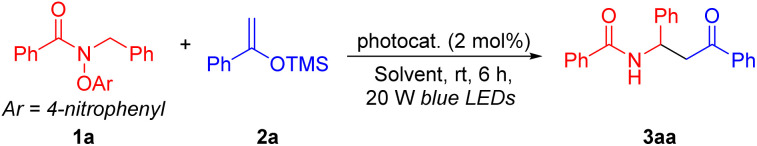
Reaction conditions: 1a (0.1 mmol, 1.0 equiv.), 2a (0.15 mmol, 1.5 equiv.), PC (2 mol%), rt, 6 h.
Assay yields determined by 1H NMR spectroscopy of the crude reaction mixtures using CH2Br2 as an internal standard.
1.5 : 1 ratio of 1a to 2a.
Isolated yield after chromatographic purification.
Without blue LEDs.
