Abstract
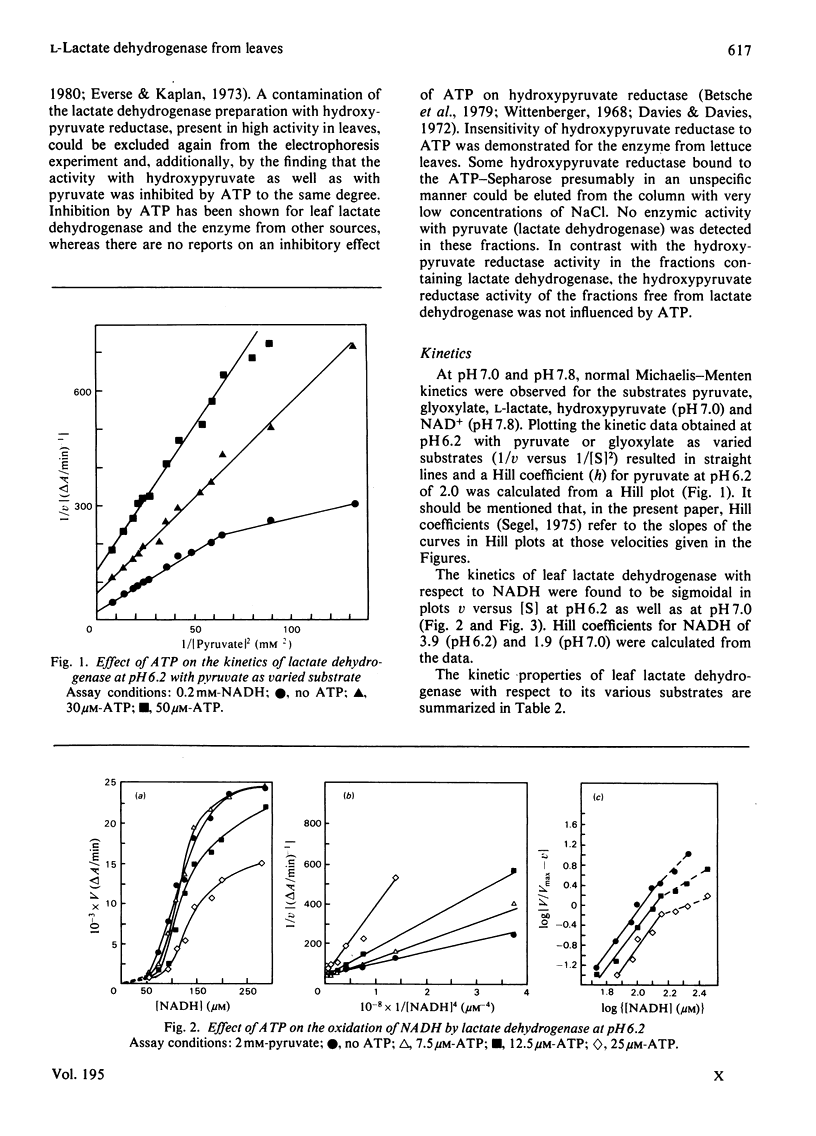
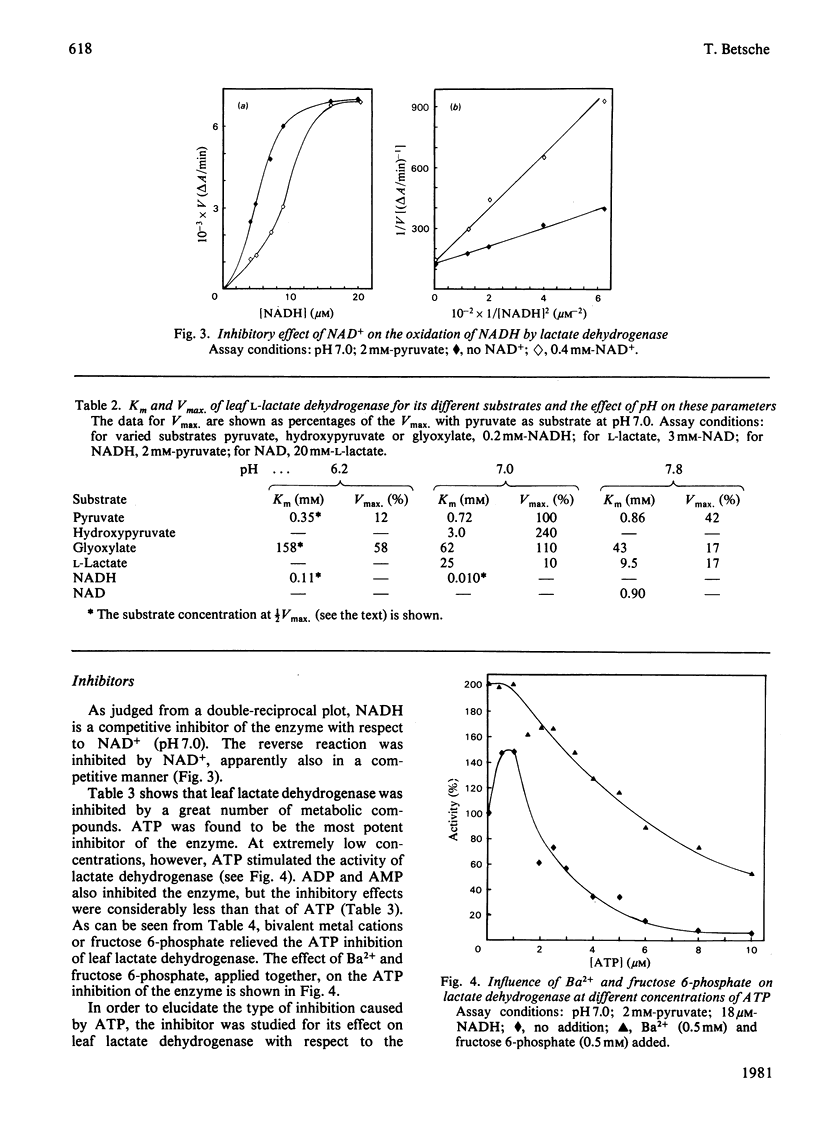
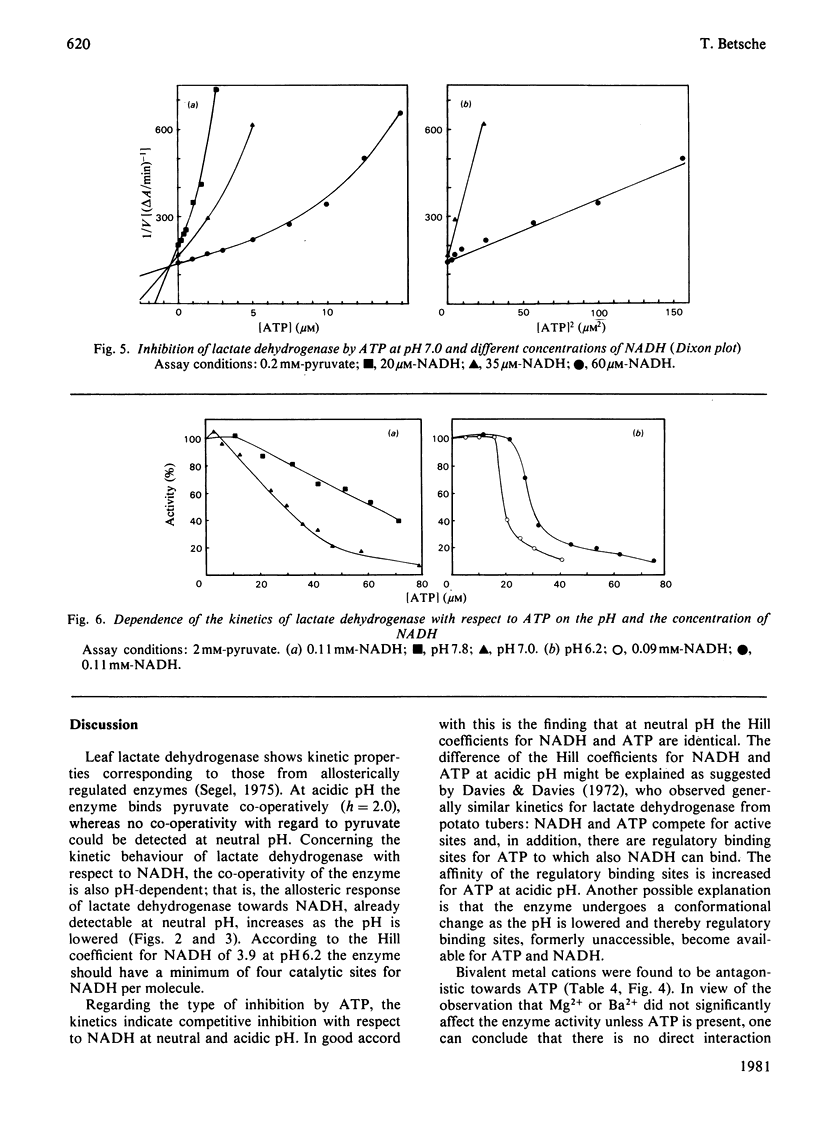
1. L-Lactate dehydrogenase from lettuce (Lactuca sativa) leaves was purified to electrophoretic homogeneity by affinity chromatography. 2. In addition to its NAD(H)-dependent activity with L-lactate and pyruvate, the enzyme also catalyses the reduction of hydroxypyruvate and glyoxylate. The latter activities are not due to a contamination of the enzyme preparations with hydroxypyruvate reductase. 3. The enzyme shows allosteric properties that are markedly by the pH. 4. ATP is a potent inhibitor of the enzyme. The kinetic data suggest that the inhibition by ATP is competitive with respect to NADH at pH 7.0 and 6.2. The existence of regulatory binding sites for ATP and NADH is discussed. 5. Bivalent metal cations and fructose 6-phosphate relieve the ATP inhibition of the enzyme. 6. A function of leaf L-lactate dehydrogenase is proposed as a component of the systems regulating the cellular pH and/or controlling the concentration of reducing equivalents in the cytoplasm of leaf cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies D. D., Davies S. Purification and properties of L(+)-lactate dehydrogenase from potato tubers. Biochem J. 1972 Oct;129(4):831–839. doi: 10.1042/bj1290831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber P. J., Frederick S. E., Tolbert N. E. Enzymes related to lactate metabolism in green algae and lower land plants. Plant Physiol. 1974 Feb;53(2):167–170. doi: 10.1104/pp.53.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis L., Schmidt C. N. Affinity chromatography of potato lactate dehydrogenase [proceedings]. Biochem Soc Trans. 1977;5(6):1767–1770. doi: 10.1042/bst0051767. [DOI] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz G. W., Eber S., Kessler W., Krietsch H., Krietsch W. K. Isolation of phosphoglycerate kinases by affinity chromatography. Eur J Biochem. 1978 Apr 17;85(2):493–501. doi: 10.1111/j.1432-1033.1978.tb12265.x. [DOI] [PubMed] [Google Scholar]
- Oba K., Murakami S., Uritani I. Partial purification and characterization of L-lactate dehydrogenase isozymes from sweet potato roots. J Biochem. 1977 May;81(5):1193–1201. [PubMed] [Google Scholar]
- Poerio E., Davies D. D. A comparison of potato and vertebrate lactate dehydrogenases. Biochem J. 1980 Nov 1;191(2):341–348. doi: 10.1042/bj1910341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe G. M. Catalytic properties of three lactate dehydrogenases from potato tubers (Solanum tuberosum). Arch Biochem Biophys. 1974 May;162(1):17–21. doi: 10.1016/0003-9861(74)90100-3. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L. Kinetic studies on the inhibition of a (D(-)-specific lactate dehydrogenase by adenosine triphosphate. J Biol Chem. 1968 Jun 10;243(11):3067–3075. [PubMed] [Google Scholar]


