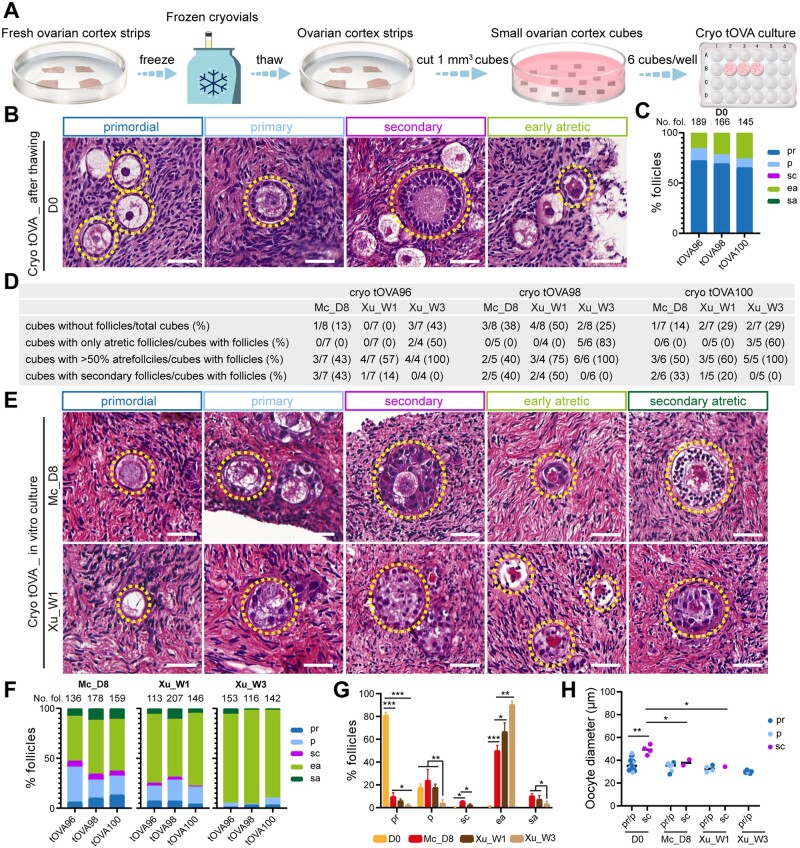
Figure 2.
Follicular distribution in cryopreserved (cryo) trans masculine ovarian cortex before and after culture. (A) Schematic workflow used to culture cryo-thawed trans masculine ovarian cortex (tOVA). (B) Hematoxylin and eosin (HE) staining of cryo-thawed tOVA prior to culture (day 0, D0), showing different types of follicles (dashed line). Scale bars are 50 µm. (C) Percentage of follicular distribution in cryo-thawed tOVA per donor prior to culture (D0). In the top of the graph are the total number of follicles counted per donor. (D) Characteristics of the tOVA tissue cubes analyzed per donor regarding follicles. (E) HE staining of cryo-thawed tOVA after culture, showing different types of follicles (dashed line). Scale bars are 50 µm. (F) Percentage of follicular distribution in cryo-thawed tOVA per donor after culture. In the top of the graph are the total number of follicles counted per donor. (G) Percentage of follicular distribution in cryo-thawed tOVA per follicular stage before and after culture. The results shown are mean ± standard deviation from three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001. (H) Oocyte diameter was measured in pr/p follicles and sc follicles in cryo-thawed tOVA before and after culture. The results shown are mean ± standard deviation; *P < 0.05, **P < 0.01, compared to sc follicles at D0. Mc_D8, culture after McLaughlin et al. (2018) for 8 days; Xu_W1/W3, culture after Xu et al. (2021) for 1/3 weeks; pr, primordial; p, primary; sc, secondary; ea, early atretic; sa, secondary atretic.