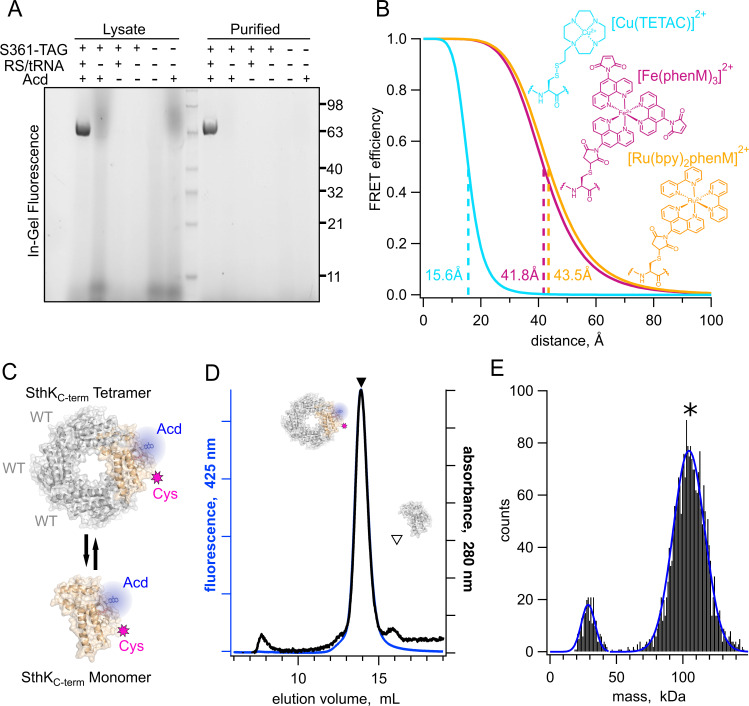
Figure 2. Expression, purification, and analysis of tetrameric SthKCterm.
(A) In-gel protein fluorescence showing selective Acd incorporation into SthKCterm in the absence and presence of S361Acd TAG site, Acd aminoacyl tRNA synthetase/tRNA (RS/tRNA), and unnatural amino acid Acd. (B) Structures of cysteine modified by acceptor compounds [Cu(TETAC)]2+ (cyan), [Fe(phenM)3]2+ (magenta), and [Ru(bpy)2phenM]2+ (orange) along with their corresponding Förster curves of FRET efficiency as a function of distance from Acd, and their R0 values specified and marked with dashed lines. (C) SthKCterm cartoon as tetramer and monomer showing WT subunits in gray and cysteine-containing Acd-labeled subunits in tan. (D) SEC traces (absorbance at 280 nm in black and 425 nm fluorescence emission for Acd in blue) of isolated WT-Acd-heterotetrameric protein (closed triangle) vs monomeric WT protein (open triangle). (E) Mass photometry histogram data showing primarily tetramers (*), with single Gaussian fits (blue, 29±7.3 kDa and 104.5±16.8 kDa , means ± SD).