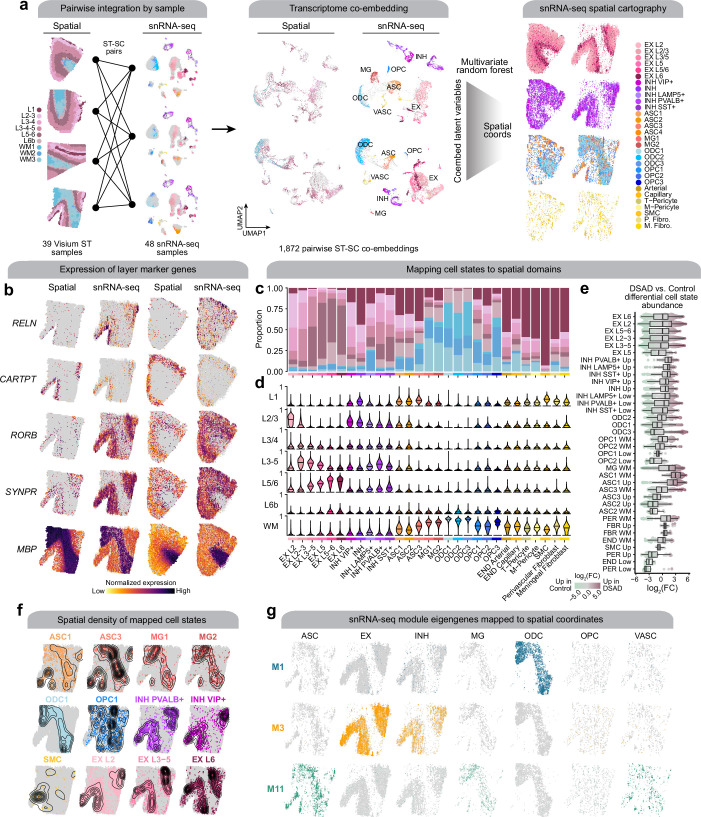
Extended Data Fig. 6. Systematic integration of spatial and single-nucleus expression profiles.
a, Pairwise integration of samples from spatial and single-nucleus transcriptomics (left). For all possible pairs of ST + snRNA-seq samples, we constructed a transcriptomic co-embedding (middle) and used a multivariate random forest (CellTrek45) to predict the spatial coordinates of snRNA-seq cells in the given spatial context. The snRNA-seq dataset is shown on the right projected into two different spatial contexts (left: control sample; right: DSAD sample), split by major cell lineages and colored by cell annotations. b, Spatial feature plots of selected layer-specific marker genes, shown side-by-side in the ST dataset and the snRNA-seq dataset projected into the spatial context for one DSAD sample (left) and one control sample (right). c, Proportion of nuclei from each snRNA-seq cluster mapped to the spatial domains defined by the ST clustering. d, Distribution of spatial domain mapping probabilities for nuclei from each of the snRNA-seq clusters. e, Box and whisker plots showing differential cell composition between disease and control. Groups are organized on the y-axis by major cell types and ordered by median fold-change values within each cell type. Box boundaries and lines correspond to the IQR and median, respectively. Whiskers extend to the lowest or highest data points that are no further than 1.5 times the IQR from the box boundaries. Each data point represents a single-cell neighborhood from Milo; the number of cell neighborhoods per cluster is shown in Supplementary Table 5. f, Spatial density plot showing the snRNA-seq dataset in predicted spatial coordinates, highlighting selected cell populations. g, Spatial feature plots showing selected module eigengenes in the snRNA-seq dataset in predicted spatial coordinates.