Abstract
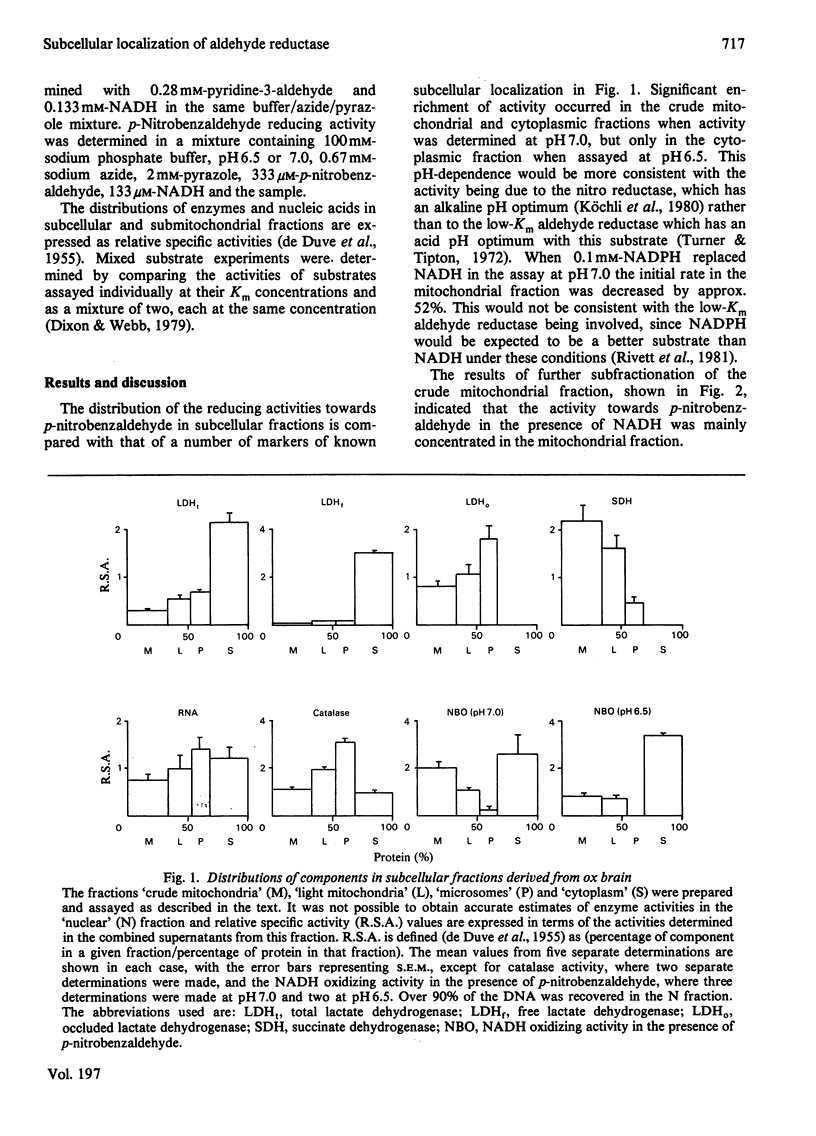
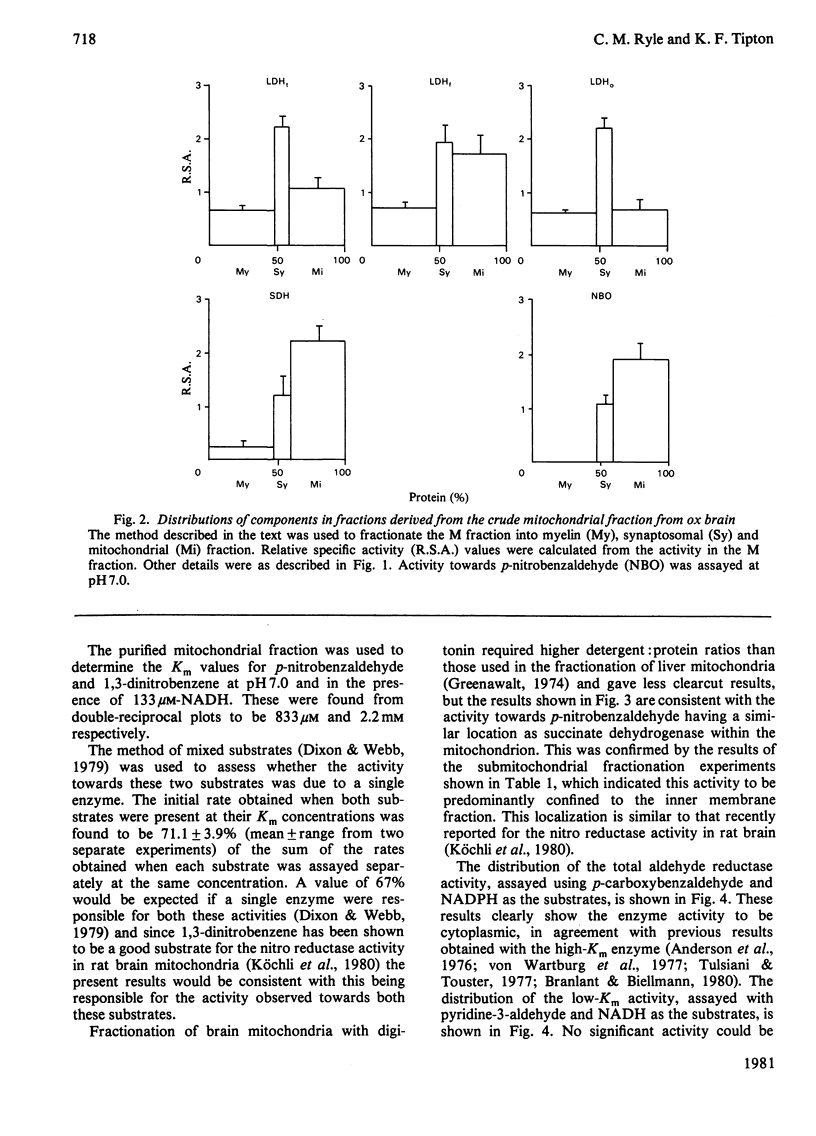
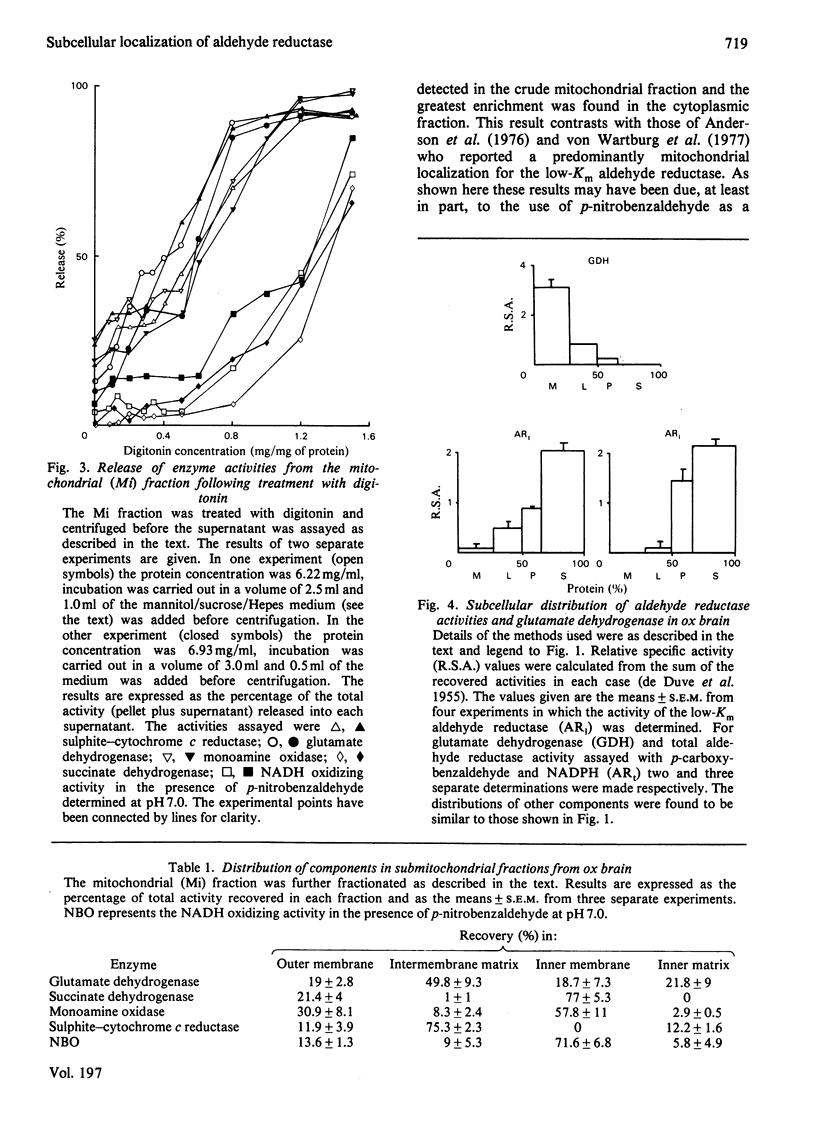
The distribution of the two principal isoenzymes of aldehyde reductase (EC 1.1.1.2) has been studied in ox brain. The more active of these, which has been termed the high-Km enzyme, has been shown to be located in the cytosol and the less abundant low-Km form has a similar localization. p-Nitrobenzaldehyde, which has been used as a substrate in previous studies, caused the reduction of NADH in the presence of the mitochondrial fraction, but mixed substrate experiments with 1,3-dinitrobenzene and the effects of pH on the activity indicate that this is due to the presence of a nitro reductase activity which has been recently described (Köchli, Wermuth & von Wartburg (1980) Biochim. Biophys. Acta 616, 133-142] rather than to the low-Km aldehyde reductase activity. Fractionation of the mitochondria indicated this activity to be largely confined to the mitochondrial inner membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant G., Biellmann J. F. Purification and some properties of aldehyde reductases from pig liver. Eur J Biochem. 1980 Apr;105(3):611–621. doi: 10.1111/j.1432-1033.1980.tb04539.x. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Goldblatt P. J., Basford R. E. Brain hexokinase. The preparation of inner and outer mitochondrial membranes. Biochemistry. 1969 Sep;8(9):3525–3532. doi: 10.1021/bi00837a007. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Goldberg R., Tipton K. F. The distribution of catechol-O-methyltransferase in pig liver and brain. Biochem Pharmacol. 1978;27(22):2623–2629. doi: 10.1016/0006-2952(78)90337-4. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. The isolation of outer and inner mitochondrial membranes. Methods Enzymol. 1974;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- Guldberg H. C., Marsden C. A. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975 Jun;27(2):135–206. [PubMed] [Google Scholar]
- Kaufman E. E., Nelson T., Goochee C., Sokoloff L. Purification and characterization of an NADP+-linked alcohol oxido-reductase which catalyzes the interconversion of gamma-hydroxybutyrate and succinic semialdehyde. J Neurochem. 1979 Mar;32(3):699–712. doi: 10.1111/j.1471-4159.1979.tb04552.x. [DOI] [PubMed] [Google Scholar]
- Köchli H. W., Wermuth B., von Wartburg J. P. Characterization of a mitochondrial NADH-dependent nitro reductase from rat brain. Biochim Biophys Acta. 1980 Dec 4;616(2):133–142. doi: 10.1016/0005-2744(80)90131-x. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The osmotically sensitive potassium and sodium compartments of synaptosomes. Biochem J. 1967 Jul;104(1):148–157. doi: 10.1042/bj1040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo G. A., Tipton K. F. The distribution of soluble and membrane-bound forms of glutaminase in pig brain. J Neurochem. 1979 Nov;33(5):1083–1084. doi: 10.1111/j.1471-4159.1979.tb05245.x. [DOI] [PubMed] [Google Scholar]
- OTSUKA S., KOBAYASHI Y. RADIOISOTOPIC ASSAY FOR MONOAMINE OXIDASE DETERMINATIONS IN HUMAN PLASMA. Biochem Pharmacol. 1964 Jul;13:995–1006. doi: 10.1016/0006-2952(64)90096-6. [DOI] [PubMed] [Google Scholar]
- Ris M. M., Deitrich R. A., Von Wartburg J. P. Inhibition of aldehyde reductase isoenzymes in human and rat brain. Biochem Pharmacol. 1975 Oct 15;24(20):1865–1869. doi: 10.1016/0006-2952(75)90405-0. [DOI] [PubMed] [Google Scholar]
- Ris M. M., von Wartburg J. P. Heterogeneity of NADPH-dependent aldehyde reductase from human and rat brain. Eur J Biochem. 1973 Aug 1;37(1):69–77. doi: 10.1111/j.1432-1033.1973.tb02958.x. [DOI] [PubMed] [Google Scholar]
- Rivett A. J., Smith I. L., Tipton K. F. The enzymes catalysing succinic semialdehyde reduction in rat brain. Biochem Pharmacol. 1981 Apr 1;30(7):741–747. doi: 10.1016/0006-2952(81)90160-x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K. F. The sub-mitochondrial localization of monoamine oxidase in rat liver and brain. Biochim Biophys Acta. 1967;135(5):910–920. doi: 10.1016/0005-2736(67)90060-0. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster Resolution and partial characterization of two aldehyde reductases of mammalian liver. J Biol Chem. 1977 Apr 25;252(8):2545–2550. [PubMed] [Google Scholar]
- Turner A. J., Illingworth J. A., Tipton K. F. Simulation of biogenic amine metabolism in the brain. Biochem J. 1974 Nov;144(2):353–360. doi: 10.1042/bj1440353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J., Tipton K. F. The characterization of two reduced nicotinamide-adenine dinucleotide phosphate-linked aldehyde reductases from pig brain. Biochem J. 1972 Dec;130(3):765–772. doi: 10.1042/bj1300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S. R., Turner A. J. Effects of the anticonvulsant sodium valproate on gamma-aminobutyrate and aldehyde metabolism in ox brain. J Neurochem. 1978 Dec;31(6):1453–1459. doi: 10.1111/j.1471-4159.1978.tb06572.x. [DOI] [PubMed] [Google Scholar]


