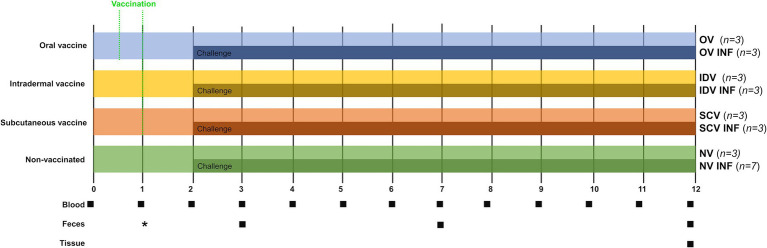
Figure 1.
Experimental design scheme. After a 2-week adaptation period, goat kids were divided into four groups and vaccinated through different routes [oral (OV), intradermal (IDV), and subcutaneous (SCV) or left unvaccinated (NV)]. Experimental (OV and IDV) and commercial vaccines (SCV—Gudair®) were used. One month after vaccination, three or seven animals from each group were orally challenged with Map (INF). A total of eight groups were formed: OV (oral vaccine), OV-INF (oral vaccine, challenged), IDV (intradermal vaccine), IDV-INF (intradermal vaccine, challenged), SCV (subcutaneous vaccine), SCV-INF (subcutaneous vaccine, challenged), NV (non-vaccinated), and NV-INF (non-vaccinated, challenged). Squares indicate sampling time points (blood, feces, and tissues) from the beginning of the study to 12 months. *Fecal samples to assure the vaccine reached the intestine were taken from the orally vaccinated animals at 3, 7, and 14 dpv.