Figure 2.
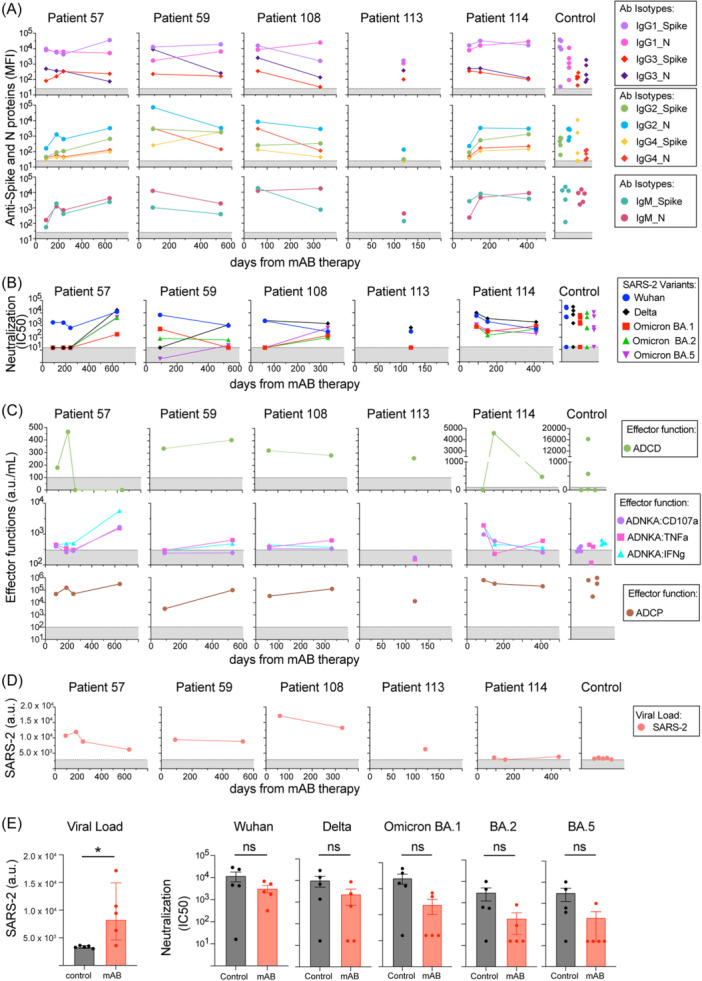
mAB‐treated patients display evidence of evolving antibody magnitudes and functions and viral load post‐mAB therapy for SARS‐CoV‐2 infection. (A) Multiplex detection of IgG1‐4 or IgM isotypes against S or N SARS‐CoV‐2 antigens was reported as mean fluorescent intensity (MFI). (B) Neutralizing antibodies against SARS‐CoV‐2 Wuhan or Delta and Omicron BA.1, BA.2, BA.5 variants. (C) Spike‐specific fc effector functions, including ADCD, ADCD, ADNKA (CD107a, TNF‐α, or IFNγ induced change), or ADCP, all reported as arbitrary units per mL of plasma (a.u./mL). (D) Viral load detection of SARS‐CoV‐2 N RNA by sensitive CRISPR assay on plasma samples, reported as arbitrary units (a.u.) of fluorescent intensity. (E) Comparison of viral load and neutralizing antibodies from mAB‐treated patients vs control group. The Gray patterned area indicated the limit of detection for each assay. All comparisons were performed between the first‐time point sample of each sample for mAB‐treated patients versus the control group with unpaired non‐parametric t‐tests.
