Figure 3.
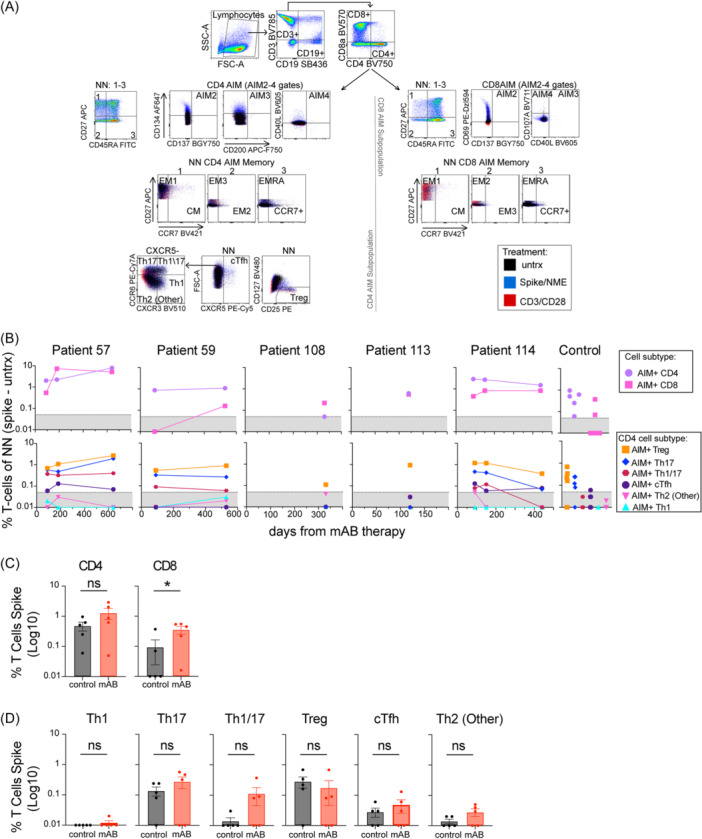
mAB‐treated patients developed spike‐specific AIM + CD4 and CD8 T‐cells, Treg, and Th17 subsets. (A) Dot blots and gating strategy for AIM panel identifying CD4 and CD8 cells in Non‐Naïve (NN, all T‐cells not CD27 + CD45RA+) expressing surface activation markers (AIM 2‐4 gates with AIM+ identified as any 2/4 activation markers expressed), CD4 T‐cell subsets (Th1, Th17, Th1/17‐like, Th2, Treg, cTfh) or CD4 or CD8 memory markers (EM1‐3, CM, EMRA). Unstimulated (untrx), spike, or non‐spike (NME) peptide pools were used for 24 h stimulation of PBMCs in this panel. (B) Normalized AIM + CD4 or CD8 AIM T‐cell, or AIM + CD4 subsets Th1, Th1/Th17, Th17, Th2 (Other), Treg, cTfh as % NN. The Gray patterned area indicated the limit of detection for AIM assay. (C) Comparison of AIM + CD4 and CD8 T‐cells from mAB‐treated patients vs control group. (D) Comparison of AIM + CD4 subsets from mAB‐treated patients vs control group. All comparisons were performed between the first‐time point sample of each sample for mAB‐treated patients versus the control group with unpaired non‐parametric t‐tests.
