Abstract
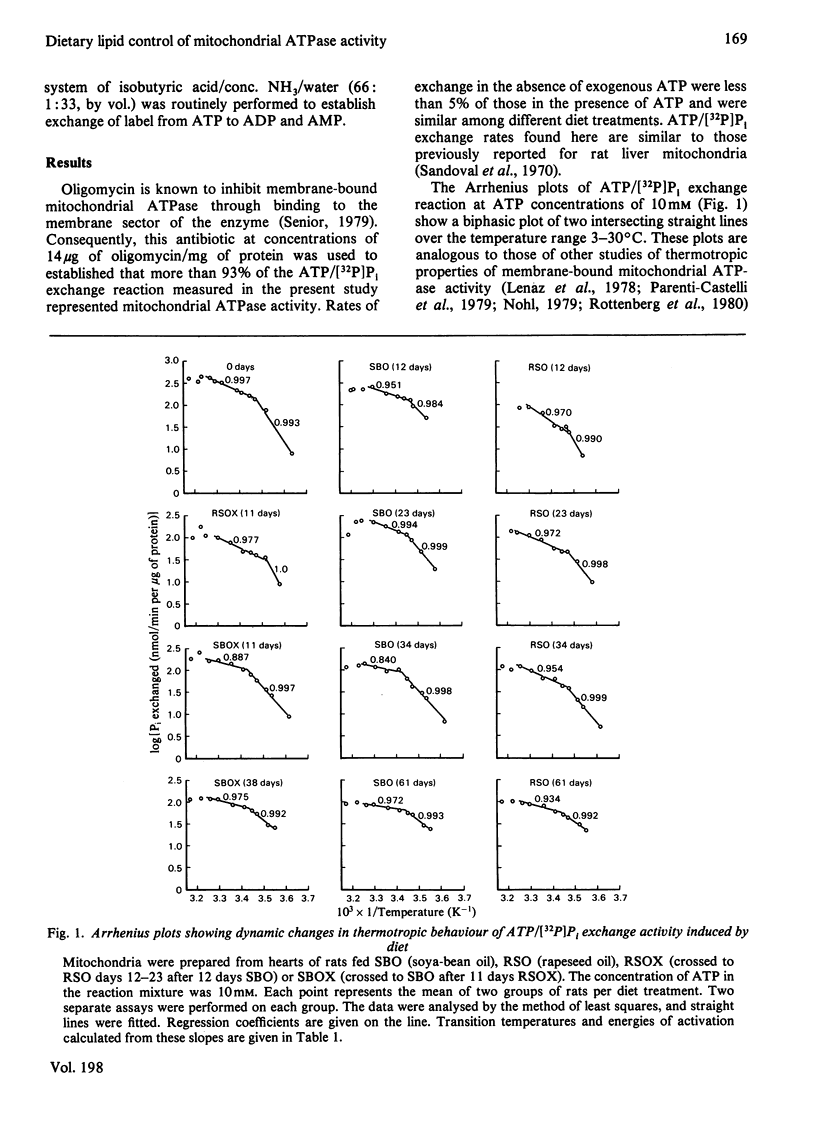
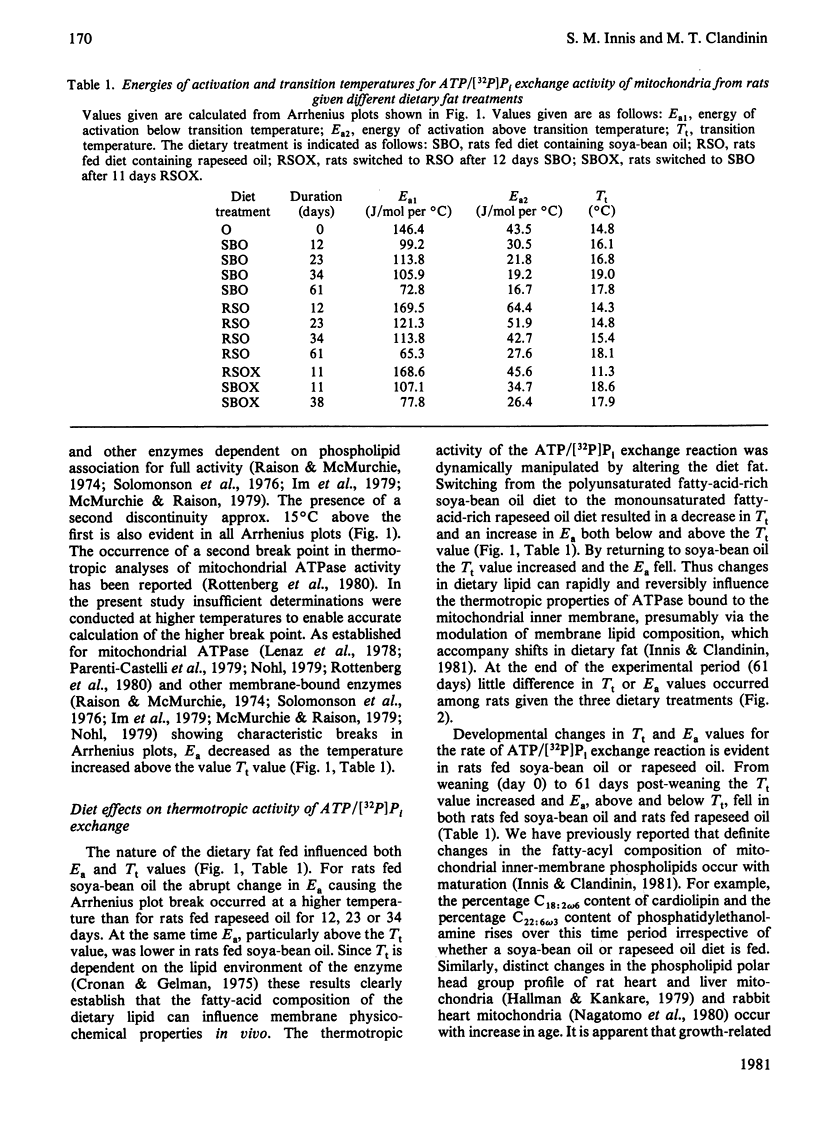
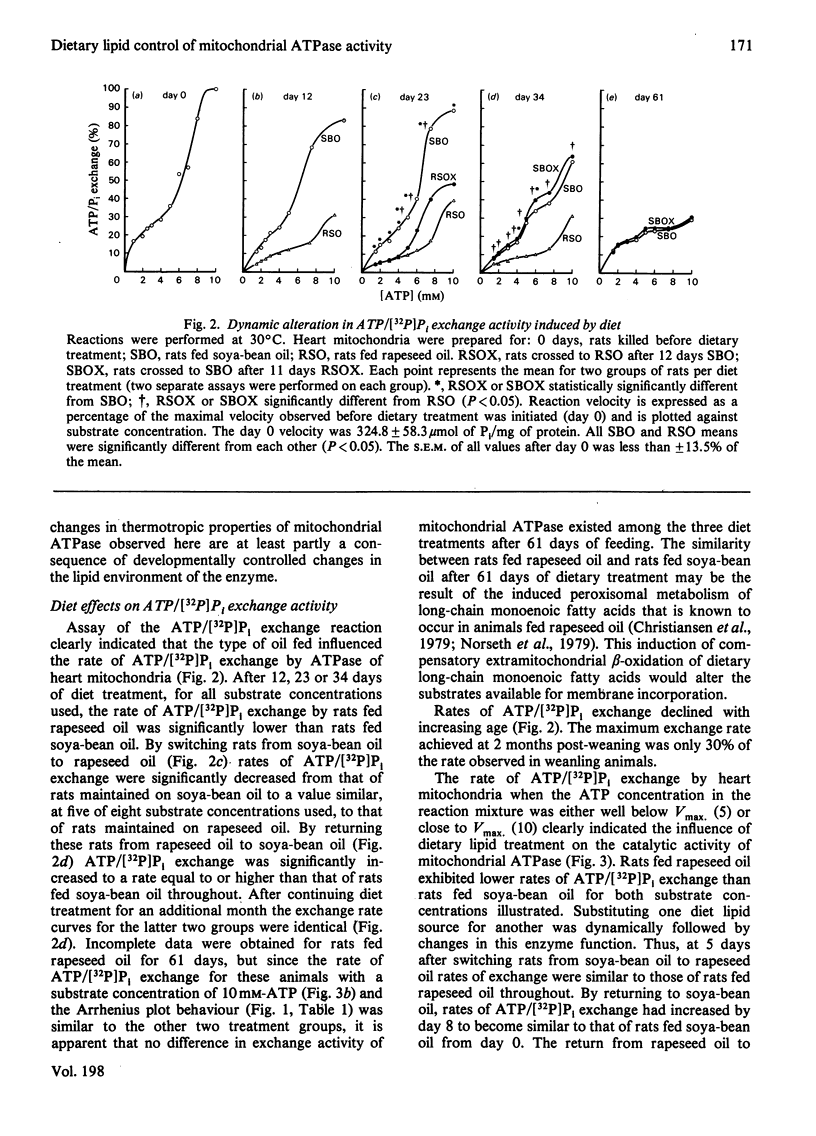
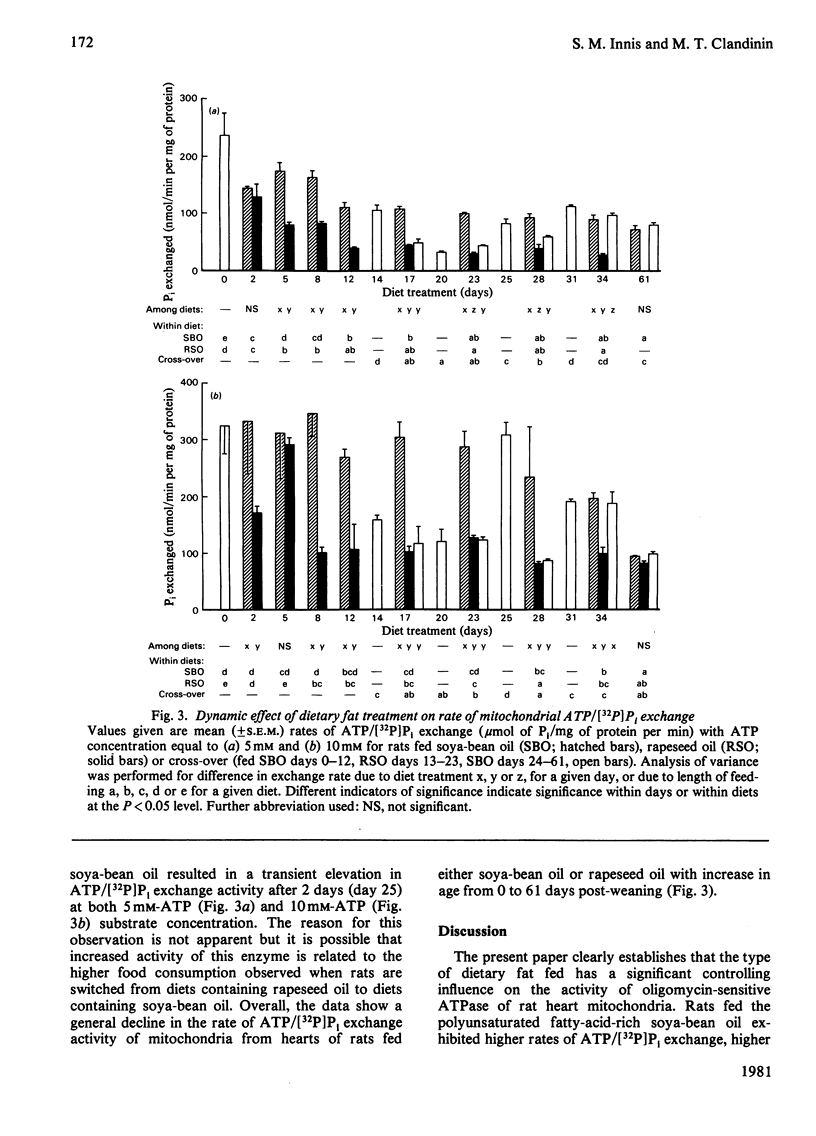
A longitudinal cross-over feeding design was used to investigate the relationship of dietary lipid composition to the membrane lipid environment and activity of mitochondrial ATPase in vivo. Rats were fed a polyunsaturated fatty-acid-rich oil (soya-bean oil) for 12 days, crossed-over to a monounsaturated fatty-acid-rich oil (rapeseed oil) for the next 11 days, then returned to soya-bean oil for 11 more days. Additional rats were fed either soya-bean oil or rapeseed oil throughout. Rats fed rapeseed oil had lower rates of ATPase-catalysed ATP/[32P]Pi exchange than rats fed soya-bean oil. Arrhenius plots showed higher transition temperature (Tt) and activation energy (Ea) for rats fed rapeseed oil. Switching from soya-bean oil to rapeseed oil was dynamically followed by changes in the thermotropic and kinetic properties of the mitochondrial ATPase exchange reaction. Returning to soya-bean oil reversed these changes. The rapid and reversible modulation of Tt caused by a change of the type of fat ingested suggests that membrane physicochemical properties are not under rigid intrinsic control but are continually modified by the profile of exogenously derived fatty acids. The studies suggest that in vivo the activity of mitochondrial ATPase is in part determined by dietary lipid via its influence on the microenvironment of the enzyme. The rapidity and ready reversibility of changes observed for this subcellular-membrane-bound enzyme suggest that dietary fatty-acid balance may be an important determinant of other membrane functions in the body.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloj B., Morero R. D., Farías R. N., Trucco R. E. Membrane lipid fatty acids and regulation of membrane-bound enzymes. Allosteric behaviour of erythrocyte Mg 2+ -ATPase, (Na + +K + )-ATPase and acetylcholinesterase from rats fed different fat-supplemented diets. Biochim Biophys Acta. 1973 Jun 7;311(1):67–79. doi: 10.1016/0005-2736(73)90255-1. [DOI] [PubMed] [Google Scholar]
- Chapman D., Gómez-Fernández J. C., Goñi F. M. Intrinsic protein--lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979 Feb 15;98(2):211–223. doi: 10.1016/0014-5793(79)80186-6. [DOI] [PubMed] [Google Scholar]
- Christiansen R. Z., Christiansen E. N., Bremer J. The stimulation of erucate metabolism in isolated rat hepatocytes by rapeseed oil and hydrogenated marine oil-containing diets. Biochim Biophys Acta. 1979 Jun 21;573(3):417–429. doi: 10.1016/0005-2760(79)90216-9. [DOI] [PubMed] [Google Scholar]
- Clandinin M. T. The role of dietary long chain fatty acids in mitochondrial structure and function. Effects on rat cardiac mitochondrial respiration. J Nutr. 1978 Feb;108(2):273–281. doi: 10.1093/jn/108.2.273. [DOI] [PubMed] [Google Scholar]
- Crain R. C., Marinetti G. V. Phospholipid topology of the inner mitochondrial membrane of rat liver. Biochemistry. 1979 May 29;18(11):2407–2414. doi: 10.1021/bi00578a041. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcans B., Jain M. K. Role of phospholipids in transport and enzymic reactions. Adv Lipid Res. 1974;12(0):147–226. doi: 10.1016/b978-0-12-024912-1.50011-9. [DOI] [PubMed] [Google Scholar]
- Gidwitz S., Pessin J. E., Weber M. J., Glaser M., Storm D. R. Effect of membrane phospholipid composition changes on adenylate cyclase activity in normal and rous-sarcoma-transformed chicken embryo fibroblasts. Biochim Biophys Acta. 1980 Mar 20;628(3):263–276. doi: 10.1016/0304-4165(80)90375-x. [DOI] [PubMed] [Google Scholar]
- Haeffner E. W., Privett O. S. Ifluence of dietary fatty acids on membrane properties and enzyme activities of liver mitochondria of normal and hypophysectomized rats. Lipids. 1975 Feb;10(2):75–81. doi: 10.1007/BF02532159. [DOI] [PubMed] [Google Scholar]
- Hallman M., Kankare P. Mitochondrial and microsomal phospholipid phosphorus metabolism during postnatal growth in rat heart and liver. Lipids. 1979 May;14(5):435–440. doi: 10.1007/BF02533458. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Houslay M. D., McGill K. A., Birdsall N. J., Metcalfe J. C., Warren G. B. Annular lipids determine the ATPase activity of a calcium transport protein complexed with dipalmitoyllecithin. Biochemistry. 1976 Sep 21;15(19):4145–4151. doi: 10.1021/bi00664a002. [DOI] [PubMed] [Google Scholar]
- Im W. B., Deutchler J. T., Spector A. A. Effects of membrane fatty acid composition on sodium-independent phenylalanine transport in Ehrlich cells. Lipids. 1979 Dec;14(12):1003–1008. doi: 10.1007/BF02533437. [DOI] [PubMed] [Google Scholar]
- Innis S. M., Clandinin M. T. Dynamic modulation of mitochondrial inner-membrane lipids in rat heart by dietary fat. Biochem J. 1981 Jan 1;193(1):155–167. doi: 10.1042/bj1930155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis S. M., Clandinin M. T. Effect of strain, sex and duration of feeding on plasma fatty acids of rats fed various dietary oils. J Nutr. 1980 May;110(5):1006–1013. doi: 10.1093/jn/110.5.1006. [DOI] [PubMed] [Google Scholar]
- Krebs J. J., Hauser H., Carafoli E. Asymmetric distribution of phospholipids in the inner membrane of beef heart mitochondria. J Biol Chem. 1979 Jun 25;254(12):5308–5316. [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. I. Lipid phase transitions. Biochim Biophys Acta. 1977 Aug 9;472(2):237–281. doi: 10.1016/0304-4157(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Lenaz G., Curatola G., Mazzanti L., Parenti-Castelli G., Landi L., Sechi A. M. A conformational model of the action of general anesthetics at the membrane level. III. Anesthetics and the properties of membrane-bound enzymes: mitochondrial ATPase. Ital J Biochem. 1978 Nov-Dec;27(6):431–449. [PubMed] [Google Scholar]
- McMurchie E. J., Raison J. K. Membrane lipid fluidity and its effect on the activation energy of membrane-associated enzymes. Biochim Biophys Acta. 1979 Jul 5;554(2):364–374. doi: 10.1016/0005-2736(79)90377-8. [DOI] [PubMed] [Google Scholar]
- Nagatomo T., Hattori K., Ikeda M., Shimada K. Lipid composition of sarcolemma, mitochondria and sarcoplasmic reticulum from newborn and adult rabbit cardiac muscle. Biochem Med. 1980 Feb;23(1):108–118. doi: 10.1016/0006-2944(80)90060-5. [DOI] [PubMed] [Google Scholar]
- Nohl H. Influence of age on thermotropic kinetics of enzymes involved in mitochondrial energy-metabolism. Z Gerontol. 1979 Jan-Feb;12(1):9–18. [PubMed] [Google Scholar]
- Norseth J., Christophersen B. O. Chain shortening of erucic acid in isolated liver cells. FEBS Lett. 1978 Apr 15;88(2):353–357. doi: 10.1016/0014-5793(78)80210-5. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Parenti-Castelli G., Sechi A. M., Landi L., Cabrini L., Mascarello S., Lenaz G. Lipid protein interactions in mitochondria. VII. A comparison of the effects of lipid removal and lipid perturbation of the kinetic properties of mitochondrial ATPase. Biochim Biophys Acta. 1979 Jul 10;547(1):161–169. doi: 10.1016/0005-2728(79)90104-x. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Raison J. K., McMurchie E. J. Two temperature-induced changes in mitochondrial membranes detected by spin labelling and enzyme kinetics. Biochim Biophys Acta. 1974 Sep 6;363(2):135–140. doi: 10.1016/0005-2736(74)90053-4. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Robertson D. E., Rubin E. The effect of ethanol on the temperature dependence of respiration and ATPase activities of rat liver mitochondria. Lab Invest. 1980 Mar;42(3):318–326. [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Sandoval F., Gómez-Puyou A., Tuena M., Chávez E., Peña A. Effect of sodium and potassium ions on mitochondrial oxidative phosphorylation. Studies with arsenate. Biochemistry. 1970 Feb 3;9(3):684–689. doi: 10.1021/bi00805a031. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Membrane lipid physical state and modulation of the Na+,Mg2+-ATPase activity in Acholeplasma laidlawii B. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1255–1259. doi: 10.1073/pnas.77.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Liepkalns V. A., Spector A. A. Changes in (Na+ + K+)-ATPase activity of Ehrlich ascites tumor cells produced by alteration of membrane fatty acid composition. Biochemistry. 1976 Feb 24;15(4):892–897. doi: 10.1021/bi00649a026. [DOI] [PubMed] [Google Scholar]
- Tuena de Gómez-Puyou M., Gavilanes M., Delaisse J. M., Gómez-Puyou A. Conformational changes of soluble mitochondrial ATPase as controlled by hydrophobic interactions within the enzyme. Biochem Biophys Res Commun. 1978 Jun 14;82(3):1028–1033. doi: 10.1016/0006-291x(78)90886-0. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Houslay M. D., Metcalfe J. C., Birdsall N. J. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature. 1975 Jun 26;255(5511):684–687. doi: 10.1038/255684a0. [DOI] [PubMed] [Google Scholar]


