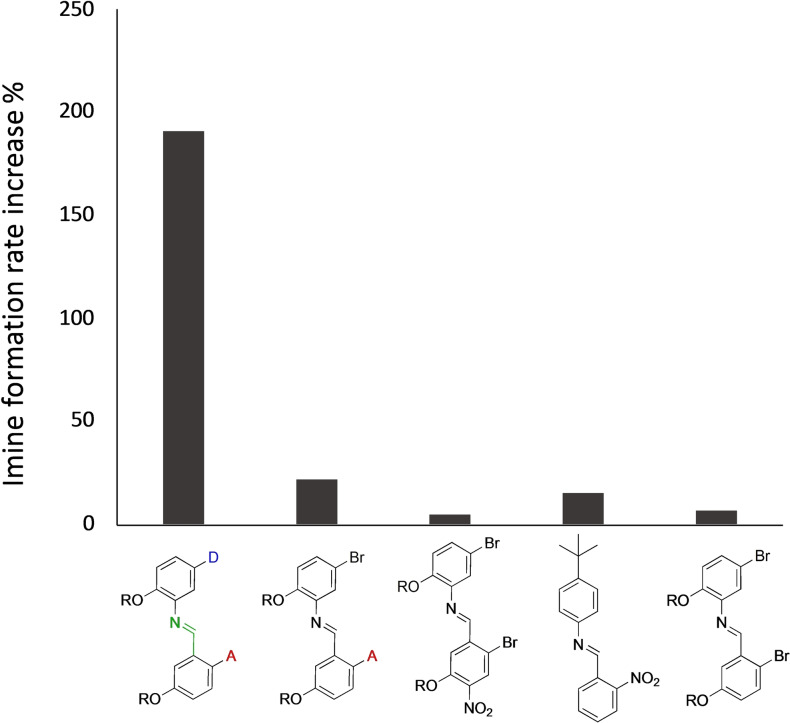
Figure 4.
Percentage of imine formation rate increase caused by the presence of an equimolar amount of phenol. The kinetic experiments were performed as detailed in the section S1.1 of the SI. A detail of the structures of the involved anilines and aldehydes is reported in Scheme S1.1. The formation rate of DA was compared with the one of DOMeA. For the other imines, 50 mM of trioctylphosphine oxide (0 mM when using aldehyde A) and 0 mM (no‐phenol control experiment) or 50 mM of 4‐bromo‐2‐(trifluoromethyl)phenol (phenol experiment) were added into the solutions. See section 3 of the SI. R = 2‐ethylhexyl.