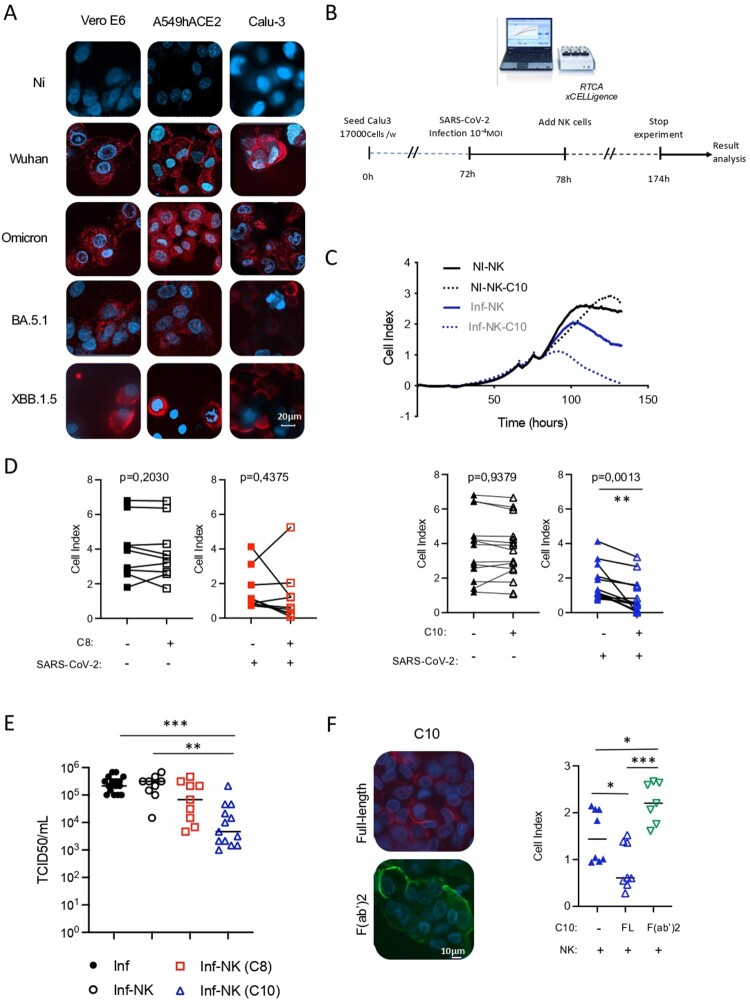
Figure 4.
Targeting capacity of SARS-CoV-2 infected cells by C8 and C10 mAbs. (A) Binding of C10 mAb to Vero E6 and lung epithelial cells infected with different SARS-CoV-2 VOCs. Non-infected cells (NI) were used as control. (B) Schematic representation of cytotoxicity assay timeline. (C) Graphical representation of cell index data obtained through the RTCA xCELLigence over-time, upon co-culture of non-infected cells (NI) or SARS-CoV2-infected Calu-3 cells (Inf) with NK cells armed or not with the C10 mAb (NK-C10 and NK, respectively). (D) ADCC capacity of C8 and C10 mAbs using infected lung epithelial cells as targets and primary NK cells as effector cells. Values are from 9 (C8) and 13 (C10) independent measurements. Significance was assessed using a paired t-test. (E) Viral titers in supernatants of infected cells in the presence of C8- and C10-armed NK cells. Values are from 13 (C8) and 9 (C10) independent measurements. Significance was assessed using one-way ANOVA with Tukey’s multiple comparisons test. (F) Assessment of the binding to SARS-CoV-2-infected cells (left) and the cytotoxic capacity (right) of the F(ab’)2 fragment of C10 mAb as compared to the full-length (FL) C10 mAb. Values are from 4 independent measurements done in duplicate.