ABSTRACT
Flaviviruses comprise a genus of enveloped, positive-sense, single-stranded RNA viruses typically transmitted between susceptible and permissive hosts by arthropod vectors. Established flavivirus threats include dengue viruses (DENV), yellow fever virus (YFV), Zika virus (ZIKV), and West Nile virus (WNV), which continue to cause over 400 million infections annually and are significant global health and economic burdens. Additionally, numerous closely related but largely understudied viruses circulate in animals and can conceivably emerge in human populations. Previous flaviviruses that were recognized to have this potential include ZIKV and WNV, which only became extensively studied after causing major outbreaks in humans. More than 50 species exist within the flavivirus genus, which can be further classified as mosquito-borne, tick-borne, insect-specific, or with no known vector. Historically, many of these flaviviruses originated in Africa and have mainly affected tropical and subtropical regions due to the ecological niche of mosquitoes. However, climate change, as well as vector and host migration, has contributed to geographical expansion, thereby posing a potential risk to global populations. For the purposes of this minireview, we focus on the mosquito-borne subgroup and highlight viruses that cause significant pathology or lethality in at least one animal species and/or have demonstrated an ability to infect humans. We discuss current knowledge of these viruses, existing animal models to study their pathogenesis, and potential future directions. Emerging viruses discussed include Usutu virus (USUV), Wesselsbron virus (WSLV), Spondweni virus (SPOV), Ilheus virus (ILHV), Rocio virus (ROCV), Murray Valley encephalitis virus (MVEV), and Alfuy virus (ALFV).
KEYWORDS: flavivirus, infectious disease, emerging viruses, species tropism, antiviral immunity
INTRODUCTION
Phylogenetic analyses have concluded that mosquito-borne flaviviruses likely originated in Africa (1, 2). With extended dry seasons, mosquitoes sought out water sources and discovered reserves stored by humans. Thus, some mosquito species, in particular Aedes aegypti, invaded and infested human-populated regions and subsequently developed a biting preference for humans (3). The urban transmission cycle of mosquito-vectored viruses was thereby initiated, and global viral spread was facilitated by insect, animal, and human migration and international trade.
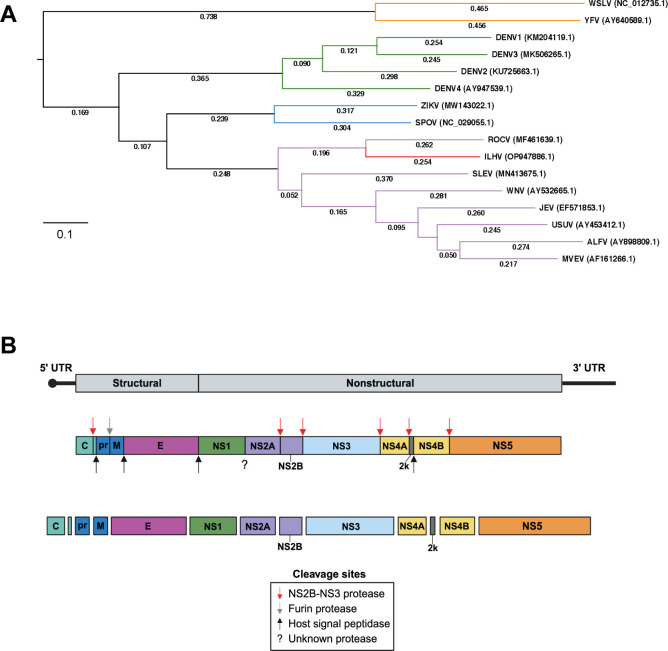
Over 3,500 mosquito species exist among 41 genera, yet flavivirus transmission is accomplished by two main genera: Aedes and Culex (4). Historically, Aedes-associated flaviviruses (YFV, DENV, and ZIKV) have caused epidemics, and Culex-associated flaviviruses (WNV and JEV) have been responsible for significant outbreaks of encephalitic disease. In general, Aedes-associated viruses cause viscerotropic or hemorrhagic symptoms, while Culex-associated viruses cause neurotropic symptoms (5). A notable exception is ZIKV, which is Aedes-associated, yet neurotropic and unique as the only flavivirus capable of being sexually transmitted between humans (6–8). Emerging Aedes-associated flaviviruses include Spondweni virus (SPOV) and Wesselsbron virus (WSLV), while emerging Culex-associated flaviviruses include Usutu virus (USUV), Ilheus virus (ILHV), Rocio virus (ROCV), and Murray Valley encephalitis virus (MVEV). Established and emerging flaviviruses that share the same mosquito vector tend to be more closely related as demonstrated by phylogenetic analysis (Fig. 1A). As climate change progresses, the ecological niches of mosquitoes will continue to expand, thereby exposing new populations to vector-borne diseases. Additionally, viral adaptation in new hosts may contribute to the emergence of novel strains that could exhibit differences in cellular or host tropism.
Fig 1.
Phylogeny and genome structure of emerging mosquito-borne flaviviruses. (A) Phylogenetic tree of the emerging flaviviruses Wesselsbron virus (WSLV), Spondweni virus (SPOV), Rocio virus (ROCV), Ilheus virus (ILHV), Usutu virus (USUV), Alfuy virus (ALFV), and Murray Valley encephalitis virus (MVEV) with reference to established flavivirus threats including yellow fever virus (YFV), dengue virus serotypes 1–4 (DENV1-4), Zika virus (ZIKV), Saint Louis encephalitis virus (SLEV), West Nile virus (WNV), and Japanese encephalitis virus (JEV). Multiple sequence alignment of full genome sequences was performed using the MAFFT algorithm, and phylogenetic analysis was performed using the randomized accelerated maximum likelihood (RAxML) program with 100 bootstrapping iterations available from DNASTAR Lasergene MegAlign Pro (Madison, WI, United States). Serocomplexes are differentially colored with YFV in orange, DENV in green, SPOV in blue, JEV in purple, and Ntaya in red. The GenBank accession numbers of the viral strains used for the alignment are indicated in parentheses. (B) Flavivirus genome organization. Flavivirus genomes are approximately 11 kb long with 5' and 3' UTRs and a 5′ terminal cap structure. The coding region contains one ORF, which is translated as a single polyprotein. Host and viral proteases cleave the polyprotein into three structural proteins (C, prM, E) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The host signal peptidase (black arrow) has cleavage activity in the endoplasmic reticulum lumen, while the viral NS2B-NS3 protease (red arrow) is active in the cytoplasm. In the Golgi apparatus, a furin protease (gray arrow) separates the pr and M proteins. Of note, the protease that cleaves between NS1 and NS2A is currently unknown.
FLAVIVIRUS GENOME ORGANIZATION AND REPLICATION
In nature, mosquito-borne flavivirus transmission initiates when an infected mosquito bites its host. As the mosquito extracts a blood meal, it releases virus and saliva that is rich in anti-inflammatory and anti-coagulant proteins. It is hypothesized that the virus first interacts with skin-resident immune cells before migrating to draining lymph nodes and gaining widespread access to additional cell types and host tissues.
Despite their differences in virulence as well as cellular and host tropism, all flaviviruses share a similar overall genome structure. Their positive-sense single-stranded RNA genome is approximately 11 kb pairs long and encodes one open reading frame (ORF) that is translated as a single polypeptide at the endoplasmic reticulum (ER) membrane (Fig. 1B). Host and viral proteases co-translationally and post-translationally cleave the polypeptide into three structural proteins and seven non-structural (NS) proteins (9). Interestingly, the ER protease that cleaves the C-terminus of NS1 remains unknown (10). The structural genes encode the proteins capsid (C), membrane (M), and envelope (E). The nonstructural (NS) genes (1, 2A, 2B, 3, 4A, 4B, and 5) encode proteins that support viral replication and evasion of the host antiviral response. Untranslated regions (UTRs) are present at the 5′ and 3′ ends of the genome, which are approximately 100 bp and 400–600 bp long, respectively. They are highly structured and contain necessary elements for viral replication (11). Flavivirus genomes have type I cap structures (m7GpppAmG) at the 5′ end and lack poly(A) tails at the 3′ end.
The flavivirus replication cycle begins with attachment to host cells and receptor engagement, followed by internalization, endosomal trafficking, capsid disassembly, initial translation and genome replication, virion assembly and maturation, and release of viral progeny. As the sole surface glycoprotein, E interacts with attachment factors on the host cell membrane to increase affinity for virus particles and receptors, which permits internalization. The host cell receptor for E remains unknown and is an ongoing area of investigation. It is possible that multiple entry factors are at play and may vary by virus, but this gap in knowledge hinders efforts to develop effective therapeutics and vaccines. While the interplay between flaviviruses and the host cell surface is not fully understood, it has been well-established that virions are internalized via clathrin-mediated endocytosis. Within endosomes, they are trafficked to the ER and undergo a process of acidification. This causes a conformational change of the virion, which enables fusion between the viral and endosomal membranes. The nucleocapsid is subsequently released into the cytoplasm and disassembly liberates the viral genome. Initial translation occurs on host cell ribosomes. Threading of the polyprotein through the ER membrane induces a curvature that leads to formation of replication organelles (ROs) or vesicle packets (VPs). These invaginations provide a replication niche shielded from detection by host pattern recognition receptors in the cytoplasm. Within the ROs, the mature NS proteins assemble into the correct structure to serve as the replication machinery known as the replication complex (RC). A double-stranded RNA (dsRNA) intermediate is produced, and the negative-sense strand subsequently serves as the template for the production of nascent positive-sense genomes.
THE EMERGENCE OF ZIKA VIRUS AND WEST NILE VIRUS AND HISTORICAL OUTBREAKS IN HUMAN POPULATIONS
Prior to causing epidemics, ZIKV and WNV were both considered emerging viral threats but were the focus of few studies. Expectedly, research of these viruses exponentially increased as they gained human relevance. ZIKV was incidentally discovered in 1947 during routine surveillance for YFV in mosquitoes and primates in Uganda and was named after the Zika forest (12). The main vectors of this virus are Aedes aegypti and Aedes albopictus. For decades, ZIKV circulation was restricted to equatorial African and Asian countries, infecting nonhuman primates (NHPs), and on rare occasions, humans causing mild febrile illness and skin rashes. In 2007, the first major ZIKV outbreak in humans occurred in the Federated States of Micronesia with 49 confirmed and 59 probable cases. This was the first record of ZIKV infection outside of Africa and Asia (13). The next emergence of ZIKV took place in French Polynesia from 2013 to 2014 with an estimated 32,000 symptomatic infections (14). The largest ZIKV epidemic occurred 2015–2016 in Brazil with an estimated 497,593 to 1,482,701 cases as reported by the Ministry of Health (15). This outbreak coincided with an upsurge of Guillain–Barré syndrome and babies born with microcephaly or other neurological defects. In 2016, the World Health Organization declared ZIKV as the source. Retrospective studies of clinical cases in French Polynesia also confirmed a causal relationship between ZIKV infections and increased incidence of Guillain–Barré syndrome in adults and microcephaly in newborn babies that were exposed during the first trimester (16, 17). Further, the first clinical case of ZIKV in the continental U.S. occurred in 2016, and local transmission was reported in Texas and Florida counties in 2017 (18–20). In recent years, ZIKV has been detected in Europe (21) and India (22), which demonstrates the significant capacity for flaviviruses to geographically expand (21, 22).
WNV was discovered in 1937 and isolated from a febrile patient in the West Nile district of Uganda (23). It is primarily vectored by Culex pipiens and utilizes wild birds, in particular crows, as amplifying hosts. Symptoms include headache and febrile illness; however, the majority of human cases are asymptomatic. During an epidemic in Israel in 1957, symptoms of neuroinvasive disease were observed for the first time in elderly patients. These symptoms were seen throughou11t sporadic outbreaks in Israel, Egypt, France, and South Africa in the following decades (24–27). Further, cases of meningoencephalitis were recorded during outbreaks in Romania and Russia in the late 1990s (28, 29). The 1999 epidemic in New York marked the emergence of WNV into the Western hemisphere and was accompanied by a striking incidence of encephalitis (30). Initial serological tests suggested that Saint Louis encephalitis virus (SLEV) was the source; however, concurrent cases of avian viral encephalitis and significant mortality in birds suggested otherwise. Although SLEV caused an outbreak of encephalitis in Missouri in 1933 with over 1,000 symptomatic cases and 200 deaths (31, 32), it was known to rarely cause death or disease in avian species (33, 34). Further testing and sequencing revealed WNV as the etiological agent (35, 36). WNV expanded across the United States, causing an estimated 7 million infections between 1999 and 2016 (37, 38). Cases continue to be reported each year and coincide with the warm weather season (39). In addition to causing neurotropic disease in humans, WNV has caused astounding lethality in bird species globally. American crows and blue jays have been particularly affected, and bird migration likely contributed to viral spread across North America.
EMERGING FLAVIVIRUS THREATS
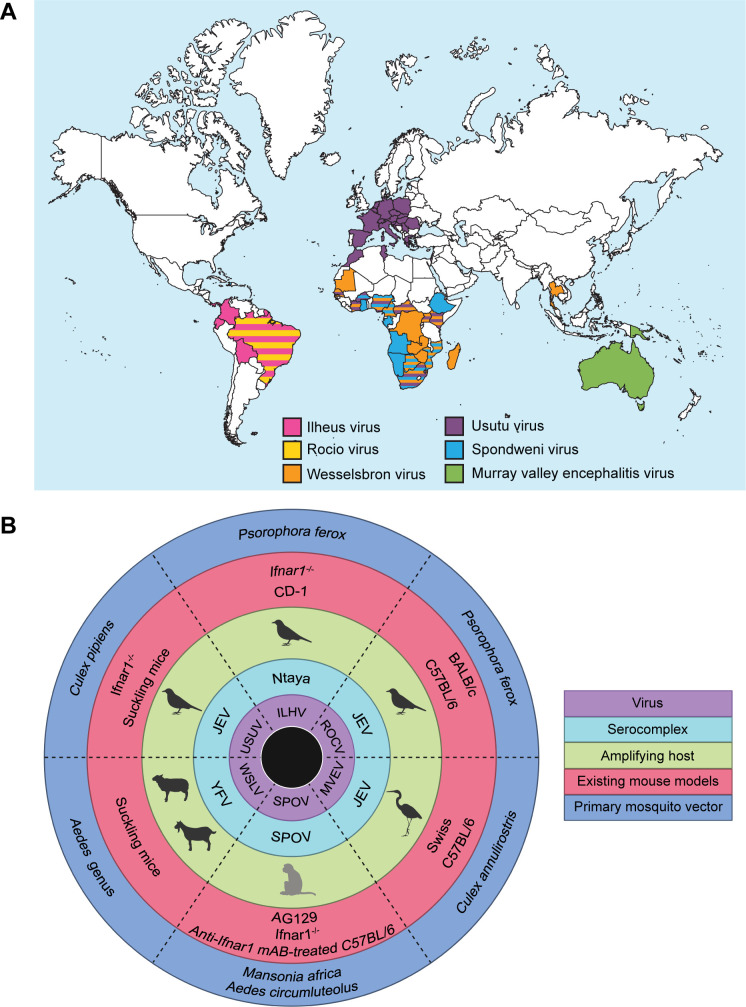
Neglected zoonotic viruses are longstanding threats to human health and emerging flaviviruses have been detected globally (Fig. 2A). They are commonly found in the same tropical and sub-tropical regions as established flaviviruses due to overlap in mosquito vectors. Major reasons to study these viral species include their prevalence and lethality in animal reservoirs and the capacity to infect humans.
Fig 2.
An overview of six emerging mosquito-borne viruses: Ilheus virus (ILHV), Rocio virus (ROCV), Wesselsbron virus (WSLV), Usutu virus (USUV), Spondweni virus (SPOV), and Murray Valley encephalitis virus (MVEV). (A) Global distribution of these emerging flaviviruses based on molecular or serological detection in humans, animals, or mosquitoes. (B) Host range of these emerging flaviviruses. The innermost ring indicates the emerging viral species. Moving outwards, the following rings indicate the serocomplex, amplifying host, existing mouse models for each virus, and the primary mosquito vector(s) responsible for transmission of each virus. While antibodies to these viruses may have been detected in a variety of animal species, only the main amplifying host(s), which manifests clinical symptoms during infection, was depicted. While the amplifying host for SPOV is not officially known, it is assumed that non-human primates (NHPs) constitute this population. NHPs are the amplifying host for ZIKV, the most closely related virus to SPOV, and rhesus macaques have been proven to be permissive after experimental inoculation (40).
Usutu virus
In 1959, USUV was discovered in South Africa during a large-scale study investigating viral species present in arthropod hosts (41). The first strain was isolated from a female Culex neavei mosquito near its eponymous river in Eswatini (41). USUV belongs to the JEV serocomplex and often co-circulates with WNV, which has a similar transmission cycle between birds and ornithophilic mosquitoes (42, 43). Culex pipiens serve as the primary vector, but USUV has also been detected in the Aedes and Mansonia genera (42, 44). These mosquitoes transmit USUV between avian amplifying reservoirs, such as Eurasian blackbirds and Great Gray owls. Systemic infection occurs in these bird species characterized by splenomegaly, hepatomegaly, and detection of USUV antigen-positive cells in the spleen, liver, brain, heart, pancreas, lung, kidney, intestine, gizzard, skeletal muscle, and bursa of Fabricius (45). The introduction of USUV into Europe was marked by the mass mortality of blackbirds in Austria in 2001 (46). Retrospective studies have since linked the true first emergence of USUV in Europe to birds in Tuscany, Italy, in 1996 (47). USUV is currently found in Africa and Europe, but only the European strains are epizootic and pathogenic in bird species (48, 49). A handful of symptomatic USUV infections have been recorded in humans with manifestations of fever, jaundice, and skin rash. In 2009, neurological symptoms presented in two immunocompromised patients in Italy, including a woman undergoing chemotherapy and a woman receiving a liver transplant (50, 51). Three additional cases of neuroinvasive disease were recorded in Croatia in 2013 (52). Antibodies against USUV have been detected in birds, bats, squirrels, wild boar, deer, and horses (45, 53–57). Due to this spread, Many European countries have instilled USUV surveillance programs with 10 countries monitoring USUV and WNV in mosquitoes. Additionally, some countries monitor wild and captive birds, boars, deer, cattle, and equids (58).
Wesselsbron virus
WSLV was first isolated in 1955 from the brain of a decomposed lamb and the blood of a febrile man in Wesselsbron, South Africa (59, 60) and was linked to abortion in domestic sheep. Today, WSLV is endemic in Africa and affects regions of South Africa in particular. It belongs to the YFV serocomplex and is mainly transmitted by Aedes mosquitoes between ruminant amplifying hosts including sheep, goats, and cattle. WSLV is teratogenic in ruminants as it can cause spontaneous abortions, congenital malformations, and stillbirths (60, 61). Adult sheep and goats often have asymptomatic infections or mild fever, whereas newborn lambs and children have an increased vulnerability marked by higher rates of mortality (62, 63). WSLV-specific antibodies have been isolated from a variety of domesticated animals, including dogs, camels, ostriches, pigs, donkeys, horses, and domestic fowls (64). Due to similar clinical manifestations, such as spontaneous abortions in livestock, WSLV cases in sheep were often initially misdiagnosed as Rift valley fever virus (RVFV), an arbovirus in the Bunyaviridae family. In humans, WSLV is subclinical or mild in most cases, but may cause acute, influenza-like illness, including fever, headaches, myalgia, and arthralgia. WSLV is restricted to Africa with the exception of WSLV isolation from mosquitoes in Thailand in 1966; however, there were no confirmed cases in any animal hosts. Only 33 human cases of WSLV have been recorded to date, the majority of which were due to laboratory exposure. One case of neuroinvasive disease occurred after direct transmission of viral suspension into the eye of a laboratory technician (59, 60, 65, 66). In Senegal, serological surveillance has indicated the presence of WSLV since the 1970s. In 2013, two novel strains of WSLV were isolated from febrile patients who were co-infected with malaria or hepatitis E. Both individuals fully recovered within a few days following peak symptoms (67).
Spondweni virus
During field collection of mosquitoes in Zululand, SPOV was isolated from Mansonia uniformus in 1955 (68). Later studies revealed that SPOV was detected in the blood of a febrile patient in Uganda 3 years prior, but was classified as ZIKV due to serological cross reactivity (69, 70). As the only two members of the SPOV serocomplex, SPOV and ZIKV are close relatives sharing 75% amino acid sequence identity (71). There is limited molecular and serological evidence confirming SPOV infection in different animal species, thus drawing conclusions about amplifying and incidental hosts is complicated. Attempts to isolate SPOV RNA or antibodies from wild birds and rodents in South Africa were unsuccessful, but antibodies were detected in a handful of cattle and sheep (72). The primary vector and preferred host remain unspecified, but the latter is likely NHPs, similar to ZIKV. SPOV has mainly been detected in field-caught Mansonia Africana and Aedes circumluteolus mosquitoes (27, 73). Current studies of vector competence have conflicting results. One group demonstrated that A. aegypti, but not Culex quinquefasciatus, transmit SPOV (74), while another group demonstrated low susceptibility and viral dissemination by A. aegypti, A. albopictus, and C. quinquefasciatus (71). Interestingly, SPOV was detected in field-caught C. quinquefasciatus in Haiti in 2016, which was the first record of SPOV outside of Africa (75). Unique from other mosquito-borne flaviviruses, the potential for sexual transmission of ZIKV and SPOV has been demonstrated (76). SPOV has not caused any human epidemics, and only six clinical cases have been described in the literature with symptoms including fever, headache, myalgia, and jaundice (69, 77). It is assumed that most infections are self-limiting and often go undiagnosed. Serological surveillance studies indicate SPOV infections in at least 10 countries in Sub-Saharan Africa (78–80).
Ilheus and Rocio viruses
As illustrated above, many flaviviruses originated in Africa; however, ILHV and ROCV were first detected in Brazil and continue to circulate throughout South and Central America. ILHV belongs to the Ntaya virus serocomplex, while ROCV is a member of the JEV serocomplex. Both viruses are neurotropic with transmission cycles involving primarily Psorophora ferox mosquito vectors and avian hosts. These viruses are closely related and share amino acid identities of approximately 75% for nonstructural protein 1 (81) and 79% for envelope protein (82). During surveillance for YFV in 1944, ILHV was isolated from Psorophora and Ochlerotatus mosquitoes in Ilheus, Bahia, Brazil (83). Prior to this, SLEV was the only neurotropic flavivirus detected in South America (84). Sporadic human infections have been recorded with one lethal case in an elderly, encephalitic patient in 2017 (85). No epizootics have been recorded in any animal species, but ILHV-specific antibodies have been detected in birds, rodents, bats, monkeys, horses, water buffalo, tortoises, and sloths (86–88). In contrast, an epidemic of human encephalitis marked the emergence of ROCV, which was named after a neighborhood in Sao Paolo, Brazil. This outbreak occurred from 1975 to 1976, causing over 100 deaths and neurological sequelae in 20% of survivors (89). The first ROCV strain was obtained from central nervous system (CNS) tissues of a male who succumbed to lethal infection within five 5 days of illness onset. Notably, ROCV has not caused additional outbreaks in humans; however, two ROCV infections were detected during a DENV outbreak in Brazil from 2011 to 2013. Both patients experienced fever, myalgia, and arthralgia, but fully recovered (90). Serological surveys indicate ROCV infection has occurred in birds, rodents bats, horses, water buffalo, and marsupials (88, 91, 92).
Murray Valley encephalitis and Alfuy viruses
In 1951, MVEV was first isolated from encephalitic patients in Australia (93) and is a major cause of arboviral infections in Australia and Papua New Guinea today (94). It belongs to the JEV serocomplex and is transmitted between water fowl, such as herons and egrets, by Culex annulirostris mosquitoes (95). Between 1 in 150 and 1 in 1,000 human cases are symptomatic, and the case fatality rate is 15%–30% (96). Symptomatic disease is characterized by fever, nausea, vomiting, rash, and confusion (97, 98) with serious neurological symptoms, including seizures in children, encephalitis, acute flaccid paralysis, and tremors (96). Interestingly, MVEV can also cause neurological disease in horses (99). Several MVEV epidemics have occurred in Australia with 45 cases in 1951, 59 cases in 1974, and 17 cases in 2011 (100). These outbreaks were preceded by heavy rainfall and regional flooding, which caused migration of infected water fowl seeking dry shelter (101, 102). Following the 1951 outbreak, serological testing in wild birds revealed that 12 out of 16 water bird species were previously infected with MVEV while only 7 out of 24 land bird species were previously infected (103). Surveillance of sentinel chickens has also shown increased seroconversion to MVEV after heavy rain seasons and therefore may serve as a useful warning for human outbreaks (104). Alfuy virus (ALFV) was previously recognized as a naturally attenuated subtype of MVEV sharing 83% amino acid identity (105). The first ALFV strain was isolated in 1966 from a pheasant in Queensland (106), and additional strains were isolated from Culex mosquitoes in 1999 (107). ALFV has not been associated with disease in humans nor other animals, but investigation of this virus in comparison to MVEV may provide insight into natural mechanisms of flavivirus attenuation. Motifs in the ALFV envelope protein have been associated with increased binding to glycosaminoglycans (GAGs), a known mechanism of flavivirus attenuation (107).
EXPERIMENTAL MOUSE MODELS FOR MOSQUITO-BORNE FLAVIVIRUS RESEARCH
Applications and limitations of cell culture models
Molecular virology studies in cell lines are a low-cost platform that allows a high degree of experimental intervention. Determining the susceptibility and permissivity of different cell lines to specific viruses suggests tissue and organismal tropisms that can be further characterized by in vivo studies. Additional cell culture experiments include viral propagation and titration, characterization of replication kinetics, generation of reporter cell lines, and screening for therapeutic candidates. While in vitro studies provide crucial preliminary insights into the molecular mechanisms of a virus in a particular cellular environment, they are unable to model how viral pathogenesis transpires in an organism with a complex immune system. Animal models provide a means for studying viral pathogenesis to determine clinical manifestation and ability to cause viremia, tissue pathology, and lethality. In addition, novel viral strains are isolated from naturally infected mosquitoes and animals. Pathological findings in animals infected in the wild have confirmed the neurotropic capacity of some viruses.
Commonly utilized mouse models
Historically, flavivirus research has been hindered by the scarcity of small animal models. Since wild-type (WT) mice are often resistant to flaviviruses, immunocompromised mice are commonly used. Some models have compromised adaptive immunity lacking functional B and T cells, while others have compromised innate immunity due to dysfunctional IFN signaling. Mice deficient in type I IFN (α, β) and/or type II IFN (γ) receptors, such as Ifnar1−/− and AG129, have been particularly useful for flavivirus research. Suckling mice, which have underdeveloped immune systems, have demonstrated a susceptibility to some flaviviruses. The emerging threats discussed in this review have been studied in many of the aforementioned mouse models and are summarized below (Fig. 2B).
USUV mouse models
Adult wild-type mice are not susceptible to USUV-related disease; however, Ifnar1−/− mice, which lack a type I IFN receptor subunit, provide a useful model for studying USUV pathogenesis and neurovirulence. The median survival time of Ifnar1−/− mice was 6 days post-infection (dpi) (108). These mice also served as a model to study the differential pathogenesis of African and European USUV clinical isolates: South Africa 1959, Senegal 2003, Spain 2009, Uganda 2010, and Netherlands 2016. For the former four strains, all Ifnar1−/− mice succumbed to lethal infection by 6 dpi and viral RNA was detected in serum, spleen, liver, heart, and brain. In contrast, mice infected with the Netherlands 2016 strain had an 88% survival rate with less inflammation and lower viral burden in tissues. Peak viremia levels were highest in mice exposed to African strains, suggesting greater pathogenesis compared with the European strains. Additionally, it was concluded that Spain 2009 and Netherlands 2016 strains were likely the result of distinct introduction events of USUV into Europe (109).
One-week-old suckling mice currently serve as the only immunocompetent mouse model to study USUV pathogenesis and neurotropism. Dose-dependent survival was reported in 1-week-old Swiss mice as 84% of animals survived following inoculation with 1e2 PFU of USUV, while only 40% of animals survived after inoculation with 1e4 PFU. This study also investigated cross-protection between USUV and WNV in 1-week-old mice. Prior infection with USUV did not prevent infection following WNV challenge, yet it did protect against disease and death (110). Another study in 1-week-old swiss mice identified massive inflammation and high levels of viral RNA in the brain, spinal cord, eye, and sciatic nerve on 6 dpi, corresponding to a 60% mortality rate. Viral RNA was also found in the liver, spleen, kidney, hindlimb muscle, and bladder (111). Regarding USUV neurotropism, neuronal apoptosis and demyelination of white matter was observed in 1-week-old NMRI mice 7–9 dpi; however, 2-week-old mice were resistant to peripheral and neuroinvasive USUV infection (112).
WSLV mouse models
African wild rodents were not susceptible to WSLV as no viremia nor symptom manifestation occurred (113). Suckling mice were found to be susceptible to WSLV by intraperitoneal (IP), intracerebral (IC), or subcutaneous (SC) injection. Peak viremia occurred 3 dpi via IC route. At this time, A. aegypti, but not C. quinquefasciatus, mosquitoes were able to transmit WSLV from infected to naïve suckling mice (114). Another study demonstrated an age-dependent susceptibility to WSLV. While adult mice successfully cleared WSLV, newborn mice succumbed to lethal infection. Infection of peritoneal macrophages isolated from mice of both ages recapitulated the in vivo findings. Electron microscopy suggested that macrophages from adult mice, but not newborn mice, phagocytosed WSLV particles (115). In 2013, WSLV infection in a black rat was recorded for the first time and isolated from brain tissue. This strain along with two human clinical isolates from the same region were passaged in suckling mice to obtain live virus. Extraction and sequencing of the genomic RNA determined that all three isolates were the same WSLV strain (67).
SPOV mouse models
Based on previous knowledge of ZIKV transmission, the potential for sexual transmission of SPOV was studied in IFNα/β/γ receptor knockout (AG129) mice. The mean survival time after SPOV injection was 10.8 days. Tissue tropism was similar to ZIKV with comparable viral titers found in serum, brain, testes, epididymitis, seminal vesicles, and eyes; however, infectious SPOV was only detected in 4% of ejaculates compared to over 60% in mice infected with ZIKV strains. Further, infectious SPOV was only detected in ejaculates collected on day 10 post-infection, whereas infectious ZIKV was detected in ejaculates during a range from days 6–14, 5–12, or 5–23 post-infection depending on the inoculation strain (76). While the potential for sexual transmission of SPOV was described in this mouse study, it is evident that the potential for sexual transmission of ZIKV is much greater. Additional studies are required to understand why ZIKV has a higher capacity for sexual transmission, but one contributing factor could be the overall higher virulence of ZIKV compared with SPOV.
Another group studied the tropism of SPOV strains Chuku 1952 and South Africa 1955 in C57BL/6 mice. Inoculation with either strain resulted in no clinical symptoms; however, administration of an IFNAR1 blocking monoclonal antibody (mAb) in C57BL/6 mice prior to SPOV exposure led to lethal or persistent infection with high levels of SPOV RNA detected in the serum, spleen, kidney, and brain at 7, 14, and 21 dpi (116). An increase in IFNγ-producing CD8+ T cells was observed 8 dpi with no change in the frequency of CD4+ T cells. This group also investigated SPOV pathogenesis in 3-week-old human STAT2 knock-in (hSTAT2 KI) mice, which was established as an immunocompetent mouse model for ZIKV (117). While SPOV NS5 was found to bind hSTAT2, it did not promote its degradation, and viral replication was not supported in these mice. Notably, passive transfer of neutralizing DENV and ZIKV mAbs protected mice treated with the IFNAR1 mAb against lethal infection and weight loss following SPOV challenge, thereby demonstrating cross-reactivity among these flaviviruses (116). In pregnant mice, SPOV was observed to cause fetal demise in Ifnar1−/− mice but not in IFNAR1 mAb-treated WT mice. SPOV RNA was detected in the placenta of both mice, but no overt fetal pathology was observed in mAb-treated WT mice. In contrast, moderate segmental necrosis of the brain and spinal cord and mild pulmonary inflammation were observed in pregnant Ifnar1−/− mice (74, 116).
ILHV and ROCV mouse models
Recently, pathogenicity of non-adapted and mouse-adapted ILHV strains was evaluated in CD-1 mice. In 4-week-old mice, intraperitoneal injection of a mouse-adapted strain caused 100% mortality with an average survival time of 5.6 days, while the non-adapted strain caused only 10% mortality. In 8-week-old mice, IP inoculation of the mouse-adapted strain caused 60% mortality, while SC inoculation caused 40% mortality. Unlike the mouse-adapted strain, the non-adapted strain was highly neurotropic with significant levels of virus detected in the brain, spinal cord, and eyes 4 dpi. Both ILHV strains caused 100% mortality in Ifnar1−/− mice by 3 dpi (118).
Four-week-old mice BALB/c mice have been established as a model to study ROCV-induced meningoencephalomyelitis. ROCV was detected in the CNS 2 h post-infection (hpi) using a high dose, and 100% mortality occurred by 9 dpi. Infiltration of lymphomononuclear cells including CD8+ T cells and F4/80+ macrophages, neuronal degeneration, and high expression of caspase-3 was observed in brain tissue during late infection (119). Production of chemokine CCL2 was found to be required for macrophage infiltration into the brain. Interactions between CCL2 and its receptor CCR2 facilitated monocyte migration into the brain and Ccr2−/− mice were found to have increased disease severity and mortality (120).
The ability of prior exposure to ILHV or SLEV to protect against lethal ROCV challenge has been studied in C57BL/6 mice. Nonlethal doses of ILHV or SLEV were administered at days 0 and 21 followed by lethal ROCV challenge on day 42. Prior infection with ILHV or SLEV conferred 100% or 70% protection against ROCV challenge, respectively (82).
MVEV and ALFV
The neuroinvasive capacity of MVEV was first demonstrated in a study utilizing Swiss mice, which determined that encephalitis was an age-dependent symptom as younger mice had an increased vulnerability (121). Further, the neurotropism of this virus was confirmed by detection in the CNS following intravenous (IV) injection of MVEV in C57BL/6 mice. In adult C57BL/6 mice, 1e8 PFU of MVEV caused 100% mortality, while 1e4-1e5 PFU caused approximately 50% mortality via intravenous route. By intracranial injection, 1e2 PFU resulted in 100% mortality (122). Separately, two strains of MVEV, which differed by one amino acid in the E glycoprotein, exhibited drastically different degrees of neuroinvasion in 3-week-old Swiss mice. The highly neuroinvasive strain was first detected in lymph nodes 24 hpi and caused 100% mortality, while the lowly neuroinvasive strain generally caused subclinical infection and was not detected in lymph nodes. While both strains entered the CNS via the olfactory lobe, the lowly neuroinvasive strain remained restricted to this region and the forebrain, while the highly neuroinvasive strain became diffusely distributed (123, 124). Further investigation revealed that a neutrophil inflammatory response accompanied CNS invasion starting five dpi and is triggered by production of the proinflammatory cytokine TNF-α and neutrophil chemoattractant N51/KC (125). Additionally, BALB/c mice were protected from lethal challenge with the highly neuroinvasive strain by previous inoculation with MVEV subviral particles (126).
While ALFV is very closely related to MVEV and JEV serocomplex members are typically neurotropic, ALFV did not invade the CNS of 3-week-old Swiss mice after peripheral inoculation (105). In contrast, 71% of 6-week-old Ifnar1−/− mice developed encephalitis following ALFV exposure. Of note, the average onset of mortality following ALFV infection was 12.1 days, which was significantly later compared to the 100% mortality observed 6 dpi with MVEV (105, 127). In comparison to MVEV, a novel attenuation marker of ALFV was discovered as a single amino acid change in domain III of the E protein, which conferred enhanced binding to GAGs and reduced neuroinvasion in 3-week-old Swiss mice (107).
ADDITIONAL ANIMAL MODELS FOR MOSQUITO-BORNE FLAVIVIRUS RESEARCH
Birds
Many bird species can serve as amplifying hosts for USUV, which necessitates an avian pathogenesis model. Juvenile chickens (1-day-old) are worthy candidates as they become viremic and shed virus detectable by oral and cloacal swabs (128). Further, heart tissue pathology aligned with myocarditis observed in USUV-infected wild birds (129). Domestic chickens have been used to study MVEV, SLEV, and WNV (130–132). Unfortunately, 2-week-old domestic geese and chickens were resistant to USUV infection (133, 134). In general, passerine birds are known to be susceptible to USUV (135, 136). Experimental inoculation of canaries resulted in 3 out of 10 animals developing viremia with brain lesions, immune cell infiltrates in the lung, splenomegaly, and pallor of liver observed at necropsy (137). In contrast, WNV was previously found to cause 100% lethality in domestic canaries, thus indicating that USUV is less pathogenic in this species (138).
In Brazil, house sparrows occupy the ROCV epidemic zone, which led investigators to evaluate their responses to experimental inoculation. Following infection, house sparrows became transiently and lowly viremic. As relatively inefficient hosts, they likely play a very minor role in ROCV transmission. Interestingly, ROCV-immune birds remained susceptible to SLEV challenge, whereas SLEV immunity protected birds against detectable viremia after ROCV challenge (139). In another study, young chicks (1–2 days old) became viremic for 4 days after ROCV exposure. Mosquitoes were allowed to feed on these chicks and two Culex species were able to transmit ROCV for the subsequent 20 days (140).
Few in vivo studies of MVEV have been reported, but low-dose experimental inoculation in little egrets, intermediate egrets, and pacific herons caused viremia. The onset of viremia occurred earlier in intermediate egrets compared with herons, which were viremic 1–4 dpi, but no difference in susceptibility was observed. Younger birds (≤2.5 months) had higher maximum viremia compared with older birds (≥ 8 months).
Sheep
Early WSLV studies in newborn lambs recorded mortality in 10 out of 37 animals. Biphasic fever, anorexia, staring coats, increased respiratory rate, lethargy, and general weakness were common symptoms preceding death. Hepatic pathology included discoloration of the liver, extensive necrosis of the parenchyma, and diffuse distribution of necrotic hepatocytes (62). This pathology varied from the well-circumscribed necrotic foci characteristic of RVFV, a bunyavirus for which WSLV is commonly misdiagnosed in ruminants (141). This group later investigated WSLV infection in adult sheep. The only clinical symptom was moderate fever, which was observed in 26 out of 33 animals. Further, microscopic liver lesions composed a milder phenotype compared with the necrosis and hepatocyte morphological change observed in newborn lambs. Ultimately, adult sheep were less susceptible to WSLV infection compared with newborn lambs, and WSLV caused less hepatic damage compared with RVFV (63).
Experimental inoculation of pregnant ewes at one-third gestation revealed that WSLV crossed the maternal–fetal interface and was neuroinvasive in fetuses. Ewes were viremic 1–5 dpi, yet viral RNA was not detected in maternal liver, spleen, nor placental-draining lymph nodes. WSLV was detected in a variety of placental and fetal tissue, and strong staining of WSLV antigen occurred throughout the fetal brain in neurons, glial cells, and neural progenitors (142). While human placental explants had previously been shown to support ZIKV replication, they did not support WSLV replication (143).
Goats
WSLV inoculation of West African dwarf goats caused 100% mortality. Clinical symptoms included weight loss, dehydration, diarrhea, and lymphocytopenia (144). In red Sokoto goats, WSLV caused 50% mortality. Fever coincided with viremia, which began 24–72 hpi and lasted 3–4 days. Tissues were collected from goats that succumbed to lethal infection, and WSLV RNA was detected in the spleen, liver, kidney, brain, lungs, adrenal glands, and mesenteric lymph nodes. Neutralizing antibodies were detected in the surviving animals (145).
Horses
Albeit rare, neurological symptoms have been observed in wild horses infected with WSLV and MVEV (99, 146). Experimental MVEV infection in 9-month-old foals resulted in minimal symptoms. Viremia occurred in 5 out of 11 horses accompanied by transient fever and reduced appetite. All horses developed hemagglutination-inhibiting antibodies and were ultimately deemed unlikely amplifying hosts (147). Experimental infection of horses with WSLV has not yet been accomplished. Survey studies of USUV in wild horses have been performed in European countries, including Croatia, Poland, Israel, France, and Spain but inoculation of horses in a laboratory setting has yet to be done (53, 148–151).
Non-human primates
The official amplifying host of SPOV is unknown but is speculated to be non-human primates (NHPs). One group investigated SPOV infection in two macaque species. While rhesus macaques were permissive, cynomolgus macaques restricted SPOV infection; however, SPOV efficiently replicated in primary skin fibroblasts isolated from both species. Additional experiments investigated the protective immunity provided by SPOV or ZIKV infection in heterologous challenges. SPOV-exposed rhesus macaques were susceptible to subsequent ZIKV challenge; however, ZIKV-immune macaques were protected against subsequent SPOV challenge (40). NHP experiments have not been performed for any other aforementioned emerging flavivirus.
CONCLUSIONS
Species differences likely contribute to variation in flavivirus susceptibility
Whether a flavivirus replicates and progresses to mild or systemic infection in a particular animal likely depends on virus-specific mechanisms of immune antagonism and host-specific restriction factors. For example, ZIKV NS5 binds and degrades human STAT2, thereby antagonizing the innate immune response; however, it does not interact with mouse STAT2 nor cause disease in mice. The emerging mosquito-borne flaviviruses described above demonstrate lethality in certain animal species but are generally overcome by immunocompetent human hosts. USUV, ILHV, ROCV, MVEV, and ALFV all utilize avian amplifying hosts that have simpler immune systems compared to humans and mice. Birds only have one type of polymorphonuclear leukocyte, yet they have nucleated thrombocytes that are involved in innate immunity. Additionally, birds have reduced antibody diversity due to a simplified process of immunoglobulin gene rearrangement (152, 153). WSLV mainly affects ruminant animals, which have a strikingly high proportion of gamma/delta (γδ) T cells compared to humans and mice (154). Fetal and newborn lambs are most vulnerable to lethal infection, which might be explained by an immaturity of the immune system and permissivity of fetal tissues that is lost with further development. While there are major species differences, subtle evolutionary differences can also have large effects, as seen between human and mouse STAT2 in ZIKV infection. Further studies are required to uncover these nuances.
Studying emerging flaviviruses is a valuable preventative measure
Flaviviruses continue to cause millions of infections each year with very few effective vaccines and therapeutics available. Neglected flaviviruses have been detected in a variety of animal species, some of which are highly lethal in certain hosts, but have yet to cause extensive human infection. As a result, their research is lowly prioritized. With increasing global temperatures, further expansion of human agriculture, and continued urbanization, regions affected by mosquito-borne viruses are projected to grow (155, 156). The high mutation rate of flaviviruses may lead to the inception of strains with increased virulence for mammalian hosts in which case prior knowledge of these viruses would facilitate reactive public health efforts. Many useful insights can be gained from studying these viruses at present, such as discovery of their cellular receptors and restriction factors. Understanding the shared and unique mechanisms employed by each virus will help explain their individual host and cell tropisms and potential for zoonotic transmission.
ACKNOWLEDGMENTS
Research in the Ploss lab is supported by grants from the National Institutes of Health (R01 AI138797, R01 AI153236, R01 AI146917, R01 AI168048, R01 AI107301, R01AI181664, and U19A171401), Open Philanthropy and Princeton University. A.N.N. is supported by the National Institute of General Medicine Sciences of the National Institutes of Health under Award Numbers T32GM007388 and T32GM148739.
We thank members of the Ploss lab for critical discussion of the manuscript and assistance with figure generation. We apologize to all colleagues whose work could not be cited due to space constraints.
Contributor Information
Alexander Ploss, Email: aploss@princeton.edu.
Jacob Yount, The Ohio State University, Columbus, Ohio, USA.
Adam Bailey, University of Wisconsin-Madison, Madison, Wisconsin, USA.
REFERENCES
- 1. Gould EA, de Lamballerie X, de A. Zanotto PM, Holmes EC. 2001. Evolution, epidemiology, and dispersal of flaviviruses revealed by molecular phylogenies, p 71–103. In In Advances in virus research. Academic Press. [DOI] [PubMed] [Google Scholar]
- 2. Moureau G, Cook S, Lemey P, Nougairede A, Forrester NL, Khasnatinov M, Charrel RN, Firth AE, Gould EA, de Lamballerie X. 2015. New insights into flavivirus evolution, taxonomy and biogeographic history, extended by analysis of canonical and alternative coding sequences. PLOS ONE 10:e0117849. doi: 10.1371/journal.pone.0117849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, Ibe N, Akorli J, Otoo S, Mutebi J-P, Kriete AL, Ewing EG, Sang R, Gloria-Soria A, Powell JR, Baker RE, White BJ, Crawford JE, McBride CS. 2020. Climate and urbanization drive mosquito preference for humans. Curr Biol 30:3570–3579. doi: 10.1016/j.cub.2020.06.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foster WA, Walker ED. 2019. Chapter 15 - Mosquitoes (Culicidae), p 261–325. In Mullen GR, Durden LA (ed), Medical and veterinary Entomology, Third Edition. Academic Press. [Google Scholar]
- 5. Gaunt MW, Sall AA, Lamballerie X de, Falconar AKI, Dzhivanian TI, Gould EA. 2001. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J Gen Virol 82:1867–1876. doi: 10.1099/0022-1317-82-8-1867 [DOI] [PubMed] [Google Scholar]
- 6. Li H, Saucedo-Cuevas L, Shresta S, Gleeson JG. 2016. The neurobiology of Zika virus. Neuron 92:949–958. doi: 10.1016/j.neuron.2016.11.031 [DOI] [PubMed] [Google Scholar]
- 7. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 17:880–882. doi: 10.3201/eid1705.101939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. 2016. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission — continental United States, 2016. MMWR Morb Mortal Wkly Rep 65:215–216. doi: 10.15585/mmwr.mm6508e2 [DOI] [PubMed] [Google Scholar]
- 9. Lindenbach BD, Rice CM. 2001. Flaviviridae: the viruses and their replication, p 991–1041. In Knipe DM, Howley PM (ed), Fields virology. Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 10. Falgout B, Markoff L. 1995. Evidence that flavivirus NS1-NS2A cleavage is mediated by a membrane-bound host protease in the endoplasmic reticulum. J Virol 69:7232–7243. doi: 10.1128/JVI.69.11.7232-7243.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu L, Nomaguchi M, Padmanabhan R, Markoff L. 2008. Specific requirements for elements of the 5’ and 3’ terminal regions in flavivirus RNA synthesis and viral replication. Virology (Auckl) 374:170–185. doi: 10.1016/j.virol.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dick GWA, Kitchen SF, Haddow AJ. 1952. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520. doi: 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- 13. Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on yap Island, Federated States of Micronesia. N Engl J Med 360:2536–2543. doi: 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 14. Mallet H-P, Vial A, Musso D. 2016. Bilan de l’épidémie à virus Zika survenue en Polynésie française entre octobre 2013 et mars 2014. Bull Epidemiol Hebd. [Google Scholar]
- 15. Saúde M, Secretaria de Vigilância em Saúde . 2015. Protocolo de vigilância e resposta à ocorrência de microcefalia relacionada à infecção pelo vírus Zika: Plano Nacional de Enfrentamento à Microcefalia
- 16. Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial A-L, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra J-C, Despres P, Fournier E, Mallet H-P, Musso D, Fontanet A, Neil J, Ghawché F. 2016. Guillain-barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. The Lancet 387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet H-P. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet 387:2125–2132. doi: 10.1016/S0140-6736(16)00651-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Likos A, Griffin I, Bingham AM, Stanek D, Fischer M, White S, Hamilton J, Eisenstein L, Atrubin D, Mulay P, Scott B, Jenkins P, Fernandez D, Rico E, Gillis L, Jean R, Cone M, Blackmore C, McAllister J, Vasquez C, Rivera L, Philip C. 2016. Local mosquito-borne transmission of Zika virus - miami-dade and broward Counties, Florida, june-august 2016. MMWR Morb Mortal Wkly Rep 65:1032–1038. doi: 10.15585/mmwr.mm6538e1 [DOI] [PubMed] [Google Scholar]
- 19. Philip C, Novick CG, Novick LF. 2019. Local transmission of Zika virus in miami-dade county: the florida department of health rises to the challenge. J Public Health Manag Pract 25:277–287. doi: 10.1097/PHH.0000000000000990 [DOI] [PubMed] [Google Scholar]
- 20. Hinojosa S, Alquiza A, Guerrero C, Vanegas D, Tapangan N, Cano N, Olivarez E. 2020. Detection of a locally-acquired Zika virus outbreak in Hidalgo county, Texas through increased antenatal testing in a high-risk area. Trop Med Infect Dis 5:128. doi: 10.3390/tropicalmed5030128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giron S, Franke F, Decoppet A, Cadiou B, Travaglini T, Thirion L, Durand G, Jeannin C, L’Ambert G, Grard G, Noël H, Fournet N, Auzet-Caillaud M, Zandotti C, Aboukaïs S, Chaud P, Guedj S, Hamouda L, Naudot X, Ovize A, Lazarus C, de Valk H, Paty M-C, Leparc-Goffart I. 2019. Vector-borne transmission of Zika virus in Europe, Southern France, august 2019. Euro Surveill 24:1900655. doi: 10.2807/1560-7917.ES.2019.24.45.1900655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yadav PD, Niyas VKM, Arjun R, Sahay RR, Shete AM, Sapkal GN, D. Pawar S, Patil DY, Gupta N, Abraham P. 2022. Detection of Zika virus disease in Thiruvananthapuram, Kerala, India 2021 during the second wave of COVID‐19 pandemic. J Med Virol 94:2346–2349. doi: 10.1002/jmv.27638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smithburn KC, Hughes TP, Burke AW, Paul JH. 1940. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg s1-20:471–492. doi: 10.4269/ajtmh.1940.s1-20.471 [DOI] [Google Scholar]
- 24. Bernkopf H, Levine S, Nerson R. 1953. Isolation of West Nile virus in Israel. J Infect Dis 93:207–218. doi: 10.1093/infdis/93.3.207 [DOI] [PubMed] [Google Scholar]
- 25. Spigland I, Jasinska-Klingberg W, Hofshi E, Goldblum N. 1958. Clinical and laboratory observations in an outbreak of West Nile fever in Israel in 1957. Harefuah 54:275–280. [PubMed] [Google Scholar]
- 26. Del Giudice P, Schuffenecker I, Vandenbos F, Counillon E, Zellet H. 2004. Human West Nile virus, France. Emerg Infect Dis 10:1885–1886. doi: 10.3201/eid1010.031021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mcintosh B, Jupp P, Santos I, Meenehan G. 1976. Epidemics of west Nile and sindbis viruses in South Africa with Culex (Culex) univittatus theobald as vector. S Afr J Sci [Google Scholar]
- 28. Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. 1998. West Nile encephalitis epidemic in SouthEastern Romania. The Lancet 352:767–771. doi: 10.1016/S0140-6736(98)03538-7 [DOI] [PubMed] [Google Scholar]
- 29. Platonov AE, Shipulin GA, Shipulina OY, Tyutyunnik EN, Frolochkina TI, Lanciotti RS, Yazyshina S, Platonova OV, Obukhov IL, Zhukov AN, Vengerov YY, Pokrovskii VI. 2001. Outbreak of West Nile virus infection, volgograd Region, Russia, 1999. Emerg Infect Dis 7:128–132. doi: 10.3201/eid0701.010118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention (CDC) . 1999. Outbreak of West Nile-like viral encephalitis--New York, 1999. MMWR Morb Mortal Wkly Rep 48:845–849. [PubMed] [Google Scholar]
- 31. U. S. Treasury Department, Public Health Service . 1935. Report on the St. Louis Outbreak of Encephalitis. Pub Health Bull. Washington, D.C [Google Scholar]
- 32. Diaz A, Coffey LL, Burkett-Cadena N, Day JF. 2018. Reemergence of St. Louis encephalitis virus in the Americas. Emerg Infect Dis 24:2150–2157. doi: 10.3201/eid2412.180372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hammon WMcD, Reeves WC, Sather GE. 1951. Western equine and St. Louis encephalitis viruses in the blood of experimentally infected wild birds and epidemiological implications of findings. J Immunol 67:357–367. doi: 10.4049/jimmunol.67.4.357 [DOI] [PubMed] [Google Scholar]
- 34. Chamberlain RW, Kissling RE, Stamm DD, Sudia WD. 1957. Virus of St. Louis encephalitis in three species of wild birds1. Am J Epidemiol 65:110–118. doi: 10.1093/oxfordjournals.aje.a119853 [DOI] [PubMed] [Google Scholar]
- 35. Briese T, Jia X-Y, Huang C, Grady LJ, Lan Lipkin W. 1999. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. The Lancet 354:1261–1262. doi: 10.1016/S0140-6736(99)04576-6 [DOI] [PubMed] [Google Scholar]
- 36. Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337. doi: 10.1126/science.286.5448.2333 [DOI] [PubMed] [Google Scholar]
- 37. Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M, West Nile outbreak response working group . 2001. The outbreak of West Nile virus infection in the New York city area in 1999. N Engl J Med 344:1807–1814. doi: 10.1056/NEJM200106143442401 [DOI] [PubMed] [Google Scholar]
- 38. Ronca SE, Murray KO, Nolan MS. 2019. Cumulative incidence of West Nile virus infection, continental United States, 1999-2016. Emerg Infect Dis 25:325–327. doi: 10.3201/eid2502.180765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. CDC . 2024. Historic Data (1999-2023). West Nile Virus
- 40. Jaeger AS, Crooks CM, Weiler AM, Bliss MI, Rybarczyk S, Richardson A, Einwalter M, Peterson E, Capuano S, Barkhymer A, Becker JT, Greene JT, Freedman TS, Langlois RA, Friedrich TC, Aliota MT. 2023. Primary infection with Zika virus provides one-way heterologous protection against Spondweni virus infection in rhesus macaques. Sci Adv 9:eadg3444. doi: 10.1126/sciadv.adg3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McIntosh BM. 1985. Usutu (SA Ar 1776), nouvel arbovirus du groupe B. Int Cat Arboviruses 3:1059–1060. [Google Scholar]
- 42. Fros JJ, Miesen P, Vogels CB, Gaibani P, Sambri V, Martina BE, Koenraadt CJ, van Rij RP, Vlak JM, Takken W, Pijlman GP. 2015. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in North-Western Europe. One Health 1:31–36. doi: 10.1016/j.onehlt.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang H, Abbo SR, Visser TM, Westenberg M, Geertsema C, Fros JJ, Koenraadt CJM, Pijlman GP. 2020. Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes . Emerging Microbes & Infections 9:2642–2652. doi: 10.1080/22221751.2020.1854623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williams MC, Simpson DI, Haddow AJ, Knight EM. 1964. The isolation of West Nile virus from man and of Usutu virus from the bird-biting mosquito Mansonia aurites (theobald) in the entebbe area of Uganda. Ann Trop Med Parasitol 58:367–374. doi: 10.1080/00034983.1964.11686258 [DOI] [PubMed] [Google Scholar]
- 45. Störk T, de le Roi M, Haverkamp A-K, Jesse ST, Peters M, Fast C, Gregor KM, Könenkamp L, Steffen I, Ludlow M, Beineke A, Hansmann F, Wohlsein P, Osterhaus A, Baumgärtner W. 2021. Analysis of avian Usutu virus infections in Germany from 2011 to 2018 with focus on dsRNA detection to demonstrate viral infections. Sci Rep 11:24191. doi: 10.1038/s41598-021-03638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. 2002. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis 8:652–656. doi: 10.3201/eid0807.020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weissenböck H, Bakonyi T, Rossi G, Mani P, Nowotny N. 2013. Usutu virus, Italy, 1996. Emerg Infect Dis 19:274–277. doi: 10.3201/eid1902.121191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Becker N, Jöst H, Ziegler U, Eiden M, Höper D, Emmerich P, Fichet-Calvet E, Ehichioya DU, Czajka C, Gabriel M, Hoffmann B, Beer M, Tenner-Racz K, Racz P, Günther S, Wink M, Bosch S, Konrad A, Pfeffer M, Groschup MH, Schmidt-Chanasit J. 2012. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS ONE 7:e32604. doi: 10.1371/journal.pone.0032604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Benzarti E, Sarlet M, Franssen M, Cadar D, Schmidt-Chanasit J, Rivas JF, Linden A, Desmecht D, Garigliany M. 2020. Usutu virus epizootic in Belgium in 2017 and 2018: evidence of virus endemization and ongoing introduction events. Vect Borne Zoon Dis 20:43–50. doi: 10.1089/vbz.2019.2469 [DOI] [PubMed] [Google Scholar]
- 50. Pecorari M, Longo G, Gennari W, Grottola A, Sabbatini AM, Tagliazucchi S, Savini G, Monaco F, Simone ML, Lelli R, Rumpianesi F. 2009. First human case of Usutu virus neuroinvasive infection, Italy, august-september 2009. Euro Surveill 14:19446. doi: 10.2807/ese.14.50.19446-en [DOI] [PubMed] [Google Scholar]
- 51. Cavrini F, Gaibani P, Longo G, Pierro AM, Rossini G, Bonilauri P, Gerunda GE, Di Benedetto F, Pasetto A, Girardis M, Dottori M, Landini MP, Sambri V. 2009. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, august-september 2009. Euro Surveill 14. doi: 10.2807/ese.14.50.19448-en [DOI] [PubMed] [Google Scholar]
- 52. Santini M, Vilibic-Cavlek T, Barsic B, Barbic L, Savic V, Stevanovic V, Listes E, Di Gennaro A, Savini G. 2015. First cases of human Usutu virus neuroinvasive infection in Croatia, august–september 2013: clinical and laboratory features. J Neurovirol 21:92–97. doi: 10.1007/s13365-014-0300-4 [DOI] [PubMed] [Google Scholar]
- 53. Barbic L, Vilibic-Cavlek T, Listes E, Stevanovic V, Gjenero-Margan I, Ljubin-Sternak S, Pem-Novosel I, Listes I, Mlinaric-Galinovic G, Di Gennaro A, Savini G. 2013. Demonstration of Usutu virus antibodies in horses, Croatia. Vector-Borne Zoonotic Diseases 13:772–774. doi: 10.1089/vbz.2012.1236 [DOI] [PubMed] [Google Scholar]
- 54. Cadar D, Becker N, Campos R de M, Börstler J, Jöst H, Schmidt-Chanasit J. 2014. Usutu virus in bats, Germany, 2013. Emerg Infect Dis 20:1771–1773. doi: 10.3201/eid2010.140909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Romeo C, Lecollinet S, Caballero J, Isla J, Luzzago C, Ferrari N, García-Bocanegra I. 2018. Are tree squirrels involved in the circulation of flaviviruses in Italy? Transbound Emerg Dis 65:1372–1376. doi: 10.1111/tbed.12874 [DOI] [PubMed] [Google Scholar]
- 56. Escribano-Romero E, Lupulović D, Merino-Ramos T, Blázquez A-B, Lazić G, Lazić S, Saiz J-C, Petrović T. 2015. West Nile virus serosurveillance in pigs, wild boars, and roe deer in Serbia. Vet Microbiol 176:365–369. doi: 10.1016/j.vetmic.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 57. Musto C, Tamba M, Calzolari M, Torri D, Marzani K, Cerri J, Bonilauri P, Delogu M. 2022. Usutu virus in blackbirds (Turdus merula) with clinical signs, a case study from northern Italy. Eur J Wildl Res 68:23. doi: 10.1007/s10344-022-01572-z [DOI] [Google Scholar]
- 58. Angeloni G, Bertola M, Lazzaro E, Morini M, Masi G, Sinigaglia A, Trevisan M, Gossner CM, Haussig JM, Bakonyi T, Capelli G, Barzon L. 2023. Epidemiology, surveillance and diagnosis of Usutu virus infection in the EU/EEA, 2012 to 2021. Euro Surveill 28:2200929. doi: 10.2807/1560-7917.ES.2023.28.33.2200929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smithburn KC, Kokernot RH, Weinbren MP, Meillon B. 1957. Studies on arthropod-borne viruses of Tongaland. IX. Isolation of Wesselsbron virus from a naturally infected human being and from Aedes (Banksinella) circumluteolus Theo. S Afr J Med Sci 22:113–120. [PubMed] [Google Scholar]
- 60. Weiss KE, Haig DA, Alexander RA. 1956. Wesselsbron virus- a virus not previously described, associated with abortion in domestic animals. Onderstepoort J Vet Res:27. [Google Scholar]
- 61. Mushi EZ, Binta MG, Raborokgwe M. 1998. Wesselsbron disease virus associated with abortions in goats in Botswana. J Vet Diagn Invest 10:191. doi: 10.1177/104063879801000216 [DOI] [PubMed] [Google Scholar]
- 62. Coetzer JAW, Theodoridis A, Van Heerden A. 1978. Wesselsbron disease pathological, haematological and clinical studies in natural cases and experimentally infected new-born lambs. Onderstepoort J Vet Res 45:93–106. [PubMed] [Google Scholar]
- 63. Coetzer AW, Theodoridis A. 1982. Clinical and pathological studies in adult sheep and goats experi- mentally infected with Wesselsbron disease virus. Onderstepoort J Vet Res 49:19–22. [PubMed] [Google Scholar]
- 64. Baba SS, Fagbami AH, Ojeh CK, Olaleye OD, Omilabu SA. 1995. Wesselsbron virus antibody in domestic animals in Nigeria: retrospective and prospective studies. New Microbiol 18:151–162. [PubMed] [Google Scholar]
- 65. Weyer J, Thomas J, Leman PA, Grobbelaar AA, Kemp A, Paweska JT. 2013. Human cases of Wesselsbron disease, South Africa 2010–2011. Vector-Borne Zoonotic Dis 13:330–336. doi: 10.1089/vbz.2012.1181 [DOI] [PubMed] [Google Scholar]
- 66. Justines GA, Shope RE. 1969. Wesselsbron virus infection in a laboratory worker, with virus recovery from a throat washing. Health Lab Sci 6:46–49. [PubMed] [Google Scholar]
- 67. Diagne MM, Faye M, Faye O, Sow A, Balique F, Sembène M, Granjon L, Handschumacher P, Faye O, Diallo M, Sall AA. 2017. Emergence of Wesselsbron virus among black rat and humans in Eastern Senegal in 2013. One Health 3:23–28. doi: 10.1016/j.onehlt.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kokernot RH, Smithburn KC, Muspratt J, Hodgson B. 1957. Studies on arthropod-borne viruses of Tongaland. VIII. Spondweni virus, an agent previously unknown, isolated from Taeniorhynchus (Mansonioides) uniformis. S Afr J Med Sci 22:103–112. [PubMed] [Google Scholar]
- 69. Macnamara FN. 1954. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 48:139–145. doi: 10.1016/0035-9203(54)90006-1 [DOI] [PubMed] [Google Scholar]
- 70. Haddow AJ, Williams MC, Woodall JP, Simpson DIH, Goma LKH. 1964. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ 31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 71. Haddow AD, Nasar F, Guzman H, Ponlawat A, Jarman RG, Tesh RB, Weaver SC. 2016. Genetic characterization of Spondweni and Zika viruses and susceptibility of geographically distinct strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni Virus. PLoS Negl Trop Dis 10:e0005083. doi: 10.1371/journal.pntd.0005083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McIntosh BM, Kokernot RH, Paterson HE, DeMeillon B. 1961. Isolation of Spondweni virus from four species of culicine mosquitoes and a report of two laboratory infections with the virus. S Afr Med J 35:647–650. [PubMed] [Google Scholar]
- 73. Worth CB, Paterson HE, De Meillon B. 1961. The incidence of arthropod-borne viruses in a population of culicine mosquitoes in Tongaland, Union of South Africa (january, 1956, through april, 1960). Am J Trop Med Hyg 10:583–592. doi: 10.4269/ajtmh.1961.10.583 [DOI] [PubMed] [Google Scholar]
- 74. Jaeger AS, Weiler AM, Moriarty RV, Rybarczyk S, O’Connor SL, O’Connor DH, Seelig DM, Fritsch MK, Friedrich TC, Aliota MT. 2020. Spondweni virus causes fetal harm in Ifnar1-/- mice and is transmitted by Aedes aegypti mosquitoes. Virology (Auckl) 547:35–46. doi: 10.1016/j.virol.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. White SK, Lednicky JA, Okech BA, Morris JG, Dunford JC. 2018. Spondweni virus in field-caught Culex quinquefasciatus mosquitoes, Haiti, 2016. Emerg Infect Dis 24:1765–1767. doi: 10.3201/eid2409.171957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McDonald EM, Duggal NK, Brault AC. 2017. Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl Trop Dis 11:e0005990. doi: 10.1371/journal.pntd.0005990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wolfe M, Calisher C, Mcguire K. 1982. Spondweni virus infection in a foreign resident of upper volta. The Lancet 320:1306–1308. doi: 10.1016/S0140-6736(82)91511-2 [DOI] [PubMed] [Google Scholar]
- 78. Kokernot RH, Casaca VM, Weinbren MP, McIntosh BM. 1965. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of Angola. Trans R Soc Trop Med Hyg 59:563–570. doi: 10.1016/0035-9203(65)90159-8 [DOI] [PubMed] [Google Scholar]
- 79. Kokernot RH, Szlamp EL, Levitt J, McIntosh BM. 1965. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of the Caprivi Strip and Bechuanaland Protectorate. Trans R Soc Trop Med Hyg 59:553–562. doi: 10.1016/0035-9203(65)90158-6 [DOI] [PubMed] [Google Scholar]
- 80. Brottes H, Rickenbach A, Brès P, Salaün JJ, Ferrara L. 1966. Les arbovirus au Cameroun. Isolements à partir de moustiques. Bull World Health Organ 35:811–825. [PMC free article] [PubMed] [Google Scholar]
- 81. Saivish MV, Menezes G de L, Costa V da, Silva G da, Marques RE, Nogueira ML, Silva RAD. 2022. Predicting antigenic peptides from rocio virus NS1 protein for immunodiagnostic testing using immunoinformatics and molecular dynamics simulation. Int J Mol Sci 23:7681. doi: 10.3390/ijms23147681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Amarilla AA, Fumagalli MJ, Figueiredo ML, Lima-Junior DS, Santos-Junior NN, Alfonso HL, Lippi V, Trabuco AC, Lauretti F, Muller VD, Colón DF, Luiz JPM, Suhrbier A, Setoh YX, Khromykh AA, Figueiredo LTM, Aquino VH. 2018. Ilheus and Saint Louis encephalitis viruses elicit cross-protection against a lethal rocio virus challenge in mice. PLoS ONE 13:e0199071. doi: 10.1371/journal.pone.0199071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laemmert HW, Hughes TP. 1947. The virus of Ilhéus encephalitis; isolation, serological specificity and transmission. J Immunol Baltim Md 55:61–67. [PubMed] [Google Scholar]
- 84. Diaz LA, Ré V, Almirón WR, Farías A, Vázquez A, Sanchez-Seco MP, Aguilar J, Spinsanti L, Konigheim B, Visintin A, Garciá J, Morales MA, Tenorio A, Contigiani M. 2006. Genotype III Saint Louis encephalitis virus outbreak, Argentina, 2005. Emerg Infect Dis 12:1752–1754. doi: 10.3201/eid1211.060486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Milhim BHGA, Estofolete CF, Rocha LC da, Liso E, Brienze VMS, Vasilakis N, Terzian ACB, Nogueira ML. 2020. Fatal outcome of Ilheus virus in the cerebrospinal fluid of a patient diagnosed with encephalitis. Viruses 12:957. doi: 10.3390/v12090957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Plante JA, Plante KS, Popov VL, Shinde DP, Widen SG, Buenemann M, Nogueira ML, Vasilakis N. 2023. Morphologic and genetic characterization of Ilheus virus, a potential emergent flavivirus in the Americas. Viruses 15:195. doi: 10.3390/v15010195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pauvolid-Corrêa A, Kenney JL, Couto-Lima D, Campos ZMS, Schatzmayr HG, Nogueira RMR, Brault AC, Komar N. 2013. Ilheus virus isolation in the Pantanal, West-central Brazil. PLoS Negl Trop Dis 7:e2318. doi: 10.1371/journal.pntd.0002318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Casseb AR, Cruz AV, Jesus IS, Chiang JO, Martins LC, Silva SP, Henriques DF, Casseb LM, Vasconcelos PFC. 2014. Seroprevalence of flaviviruses antibodies in water buffaloes (Bubalus bubalis) in Brazilian Amazon. J Venom Anim Toxins incl Trop Dis 20:9. doi: 10.1186/1678-9199-20-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Souza Lopes O, Coimbra TL, de Abreu Sacchetta L, Calisher CH. 1978. Emergence of a new arbovirus disease in Brazil. I. Isolation and characterization of the etiologic agent, rocio virus. Am J Epidemiol 107:444–449. doi: 10.1093/oxfordjournals.aje.a112563 [DOI] [PubMed] [Google Scholar]
- 90. Saivish MV, da Costa VG, Rodrigues RL, Féres VCR, Montoya-Diaz E, Moreli ML. 2020. Detection of rocio virus SPH 34675 during dengue epidemics, Brazil, 2011-2013. Emerg Infect Dis 26:797–799. doi: 10.3201/2604.190487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Silva JR, Romeiro MF, de Souza WM, Munhoz TD, Borges GP, Soares OAB, de Campos CHC, Machado RZ, Silva MLCR, Faria JLM, Chávez JH, Figueiredo LTM. 2014. A Saint Louis encephalitis and rocio virus serosurvey in Brazilian horses. Rev Soc Bras Med Trop 47:414–417. doi: 10.1590/0037-8682-0117-2014 [DOI] [PubMed] [Google Scholar]
- 92. Ferreira IB, Pereira LE, Rocco IM, Marti AT, de Souza LT, Iversson LB. 1994. Surveillance of arbovirus infections in the Atlantic forest region, state of São Paulo, Brazil. I. detection of hemagglutination-inhibiting antibodies in wild birds between 1978 and 1990. Rev Inst Med Trop Sao Paulo 36:265–274. doi: 10.1590/s0036-46651994000300011 [DOI] [PubMed] [Google Scholar]
- 93. Miles JAR, Fowler MC, Howes DW. 1951. Isolation of a virus from encephalitis in South Australia: a preliminary report. Med J Aust 1:799–800. doi: 10.5694/j.1326-5377.1951.tb56527.x [DOI] [PubMed] [Google Scholar]
- 94. French EL, Anderson SG, Price AV, Rhodes FA. 1957. Murray Valley encephalitis in New Guinea. I. Isolation of Murray Valley encephalitis virus from the brain of a fatal case of encephalitis occurring in a Papuan native. Am J Trop Med Hyg 6:827–834. [PubMed] [Google Scholar]
- 95. Marshall ID. 2020. Murray Valley and kunjin encephalitis, p 151–189. In Monath Thomas P (ed), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, FL. [Google Scholar]
- 96. Knox J, Cowan RU, Doyle JS, Ligtermoet MK, Archer JS, Burrow JNC, Tong SYC, Currie BJ, Mackenzie JS, Smith DW, Catton M, Moran RJ, Aboltins CA, Richards JS. 2012. Murray Valley encephalitis: a review of clinical features, diagnosis and treatment. Med J Aust 196:322–326. doi: 10.5694/mja11.11026 [DOI] [PubMed] [Google Scholar]
- 97. Broom AK, Lindsay MDA, Plant AJ, Wright AE, Condon RJ, Mackenzie JS. 2002. Epizootic activity of Murray Valley encephalitis virus in an aboriginal community in the southeast Kimberley region of Western Australia: results of cross-sectional and longitudinal serologic studies. Am J Trop Med Hyg 67:319–323. doi: 10.4269/ajtmh.2002.67.319 [DOI] [PubMed] [Google Scholar]
- 98. Burrow JNC, Whelan PI, Kilburn CJ, Fisher DA, Currie BJ, Smith DW. 1998. Australian encephalitis in the Northern Territory: clinical and epidemiological features, 1987–1996. Aust N Z J Med 28:590–596. doi: 10.1111/j.1445-5994.1998.tb00653.x [DOI] [PubMed] [Google Scholar]
- 99. Gordon AN, Marbach CR, Oakey J, Edmunds G, Condon K, Diviney SM, Williams DT, Bingham J. 2012. Confirmed case of encephalitis caused by Murray Valley encephalitis virus infection in a horse. J VET Diagn Invest 24:431–436. doi: 10.1177/1040638711433325 [DOI] [PubMed] [Google Scholar]
- 100. Beltz LA. 2021. Chapter 7 - Murray Valley encephalitis virus, p 103–114. In Beltz LA (ed), Zika and other neglected and emerging flaviviruses. Elsevier. [Google Scholar]
- 101. Kingsford RT, Norman FI. 2002. Australian waterbirds—products of the continent’s ecology. Emu Austral Ornithol 102:47–69. doi: 10.1071/MU01030 [DOI] [Google Scholar]
- 102. Whelan PI, Jacups SP, Melville L, Broom A, Currie BJ, Krause VL, Brogan B, Smith F, Porigneaux P. 2003. Rainfall and vector mosquito numbers as risk indicators for mosquito-borne disease in central Australia. Commun Dis Intell Q Rep 27:110–116. [DOI] [PubMed] [Google Scholar]
- 103. Anderson SG. 1953. Murray Valley encephalitis: a survey of avian sera, 1951–1952. Med J Aust 1:573–576. doi: 10.5694/j.1326-5377.1953.tb84703.x [DOI] [PubMed] [Google Scholar]
- 104. Selvey LA, Johansen CA, Broom AK, Antão C, Lindsay MD, Mackenzie JS, Smith DW. 2014. Rainfall and sentinel chicken seroconversions predict human cases of Murray Valley encephalitis in the north of Western Australia. BMC Infect Dis 14:672. doi: 10.1186/s12879-014-0672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. May FJ, Lobigs M, Lee E, Gendle DJ, Mackenzie JS, Broom AK, Conlan JV, Hall RA. 2006. Biological, antigenic and phylogenetic characterization of the flavivirus Alfuy. J Gen Virol 87:329–337. doi: 10.1099/vir.0.81252-0 [DOI] [PubMed] [Google Scholar]
- 106. Doherty RL, Whitehead RH, Wetters EJ, Gorman BM. 1968. Studies of the epidemiology of arthropod-borne viru infections at Mitchell river mission, Cape York Peninsula, North Queensland. II. arbovirus infections of mosquitoes, man and domestic fowls, 1963-1966. Trans R Soc Trop Med Hyg 62:430–438. doi: 10.1016/0035-9203(68)90095-3 [DOI] [PubMed] [Google Scholar]
- 107. Westlake D, Bielefeldt-Ohmann H, Prow NA, Hall RA. 2021. Novel flavivirus attenuation markers identified in the envelope protein of alfuy virus. Viruses 13:147. doi: 10.3390/v13020147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Martín-Acebes MA, Blázquez A-B, Cañas-Arranz R, Vázquez-Calvo Á, Merino-Ramos T, Escribano-Romero E, Sobrino F, Saiz J-C. 2016. A recombinant DNA vaccine protects mice deficient in the alpha/beta interferon receptor against lethal challenge with Usutu virus. Vaccine (Auckl) 34:2066–2073. doi: 10.1016/j.vaccine.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 109. Kuchinsky SC, Hawks SA, Mossel EC, Coutermarsh-Ott S, Duggal NK. 2020. Differential pathogenesis of Usutu virus isolates in mice. PLoS Negl Trop Dis 14:e0008765. doi: 10.1371/journal.pntd.0008765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Blázquez A-B, Escribano-Romero E, Martín-Acebes MA, Petrovic T, Saiz J-C. 2015. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology (Auckl) 482:67–71. doi: 10.1016/j.virol.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 111. Clé M, Barthelemy J, Desmetz C, Foulongne V, Lapeyre L, Bolloré K, Tuaillon E, Erkilic N, Kalatzis V, Lecollinet S, Beck C, Pirot N, Glasson Y, Gosselet F, Alvarez Martinez MT, Van de Perre P, Salinas S, Simonin Y. 2020. Study of Usutu virus neuropathogenicity in mice and human cellular models. PLoS Negl Trop Dis 14:e0008223. doi: 10.1371/journal.pntd.0008223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Weissenböck H, Bakonyi T, Chvala S, Nowotny N. 2004. Experimental Usutu virus infection of suckling mice causes neuronal and glial cell apoptosis and demyelination. Acta Neuropathol 108:453–460. doi: 10.1007/s00401-004-0916-1 [DOI] [PubMed] [Google Scholar]
- 113. McIntosh BM. 1961. Susceptibility of some African wild rodents to infection with various arthropod-borne viruses. Trans R Soc Trop Med Hyg 55:63–68. doi: 10.1016/0035-9203(61)90041-4 [DOI] [PubMed] [Google Scholar]
- 114. Simasathien P, Olson LC. 1973. Factors influencing the vector potential of Aedes aegypti and Culex quinquefasciatus for Wesselsbron virus. J Med Entomol 10:587–590. doi: 10.1093/jmedent/10.6.587 [DOI] [PubMed] [Google Scholar]
- 115. Olson LC, Sithisarn P, Djinawi NK. 1975. Role of macrophages in Wesselsbron and Germiston virus infections of mice. J Infect Dis 131:119–128. doi: 10.1093/infdis/131.2.119 [DOI] [PubMed] [Google Scholar]
- 116. Salazar V, Jagger BW, Mongkolsapaya J, Burgomaster KE, Dejnirattisai W, Winkler ES, Fernandez E, Nelson CA, Fremont DH, Pierson TC, Crowe JE, Screaton GR, Diamond MS. 2019. Dengue and Zika virus cross-reactive human monoclonal antibodies protect against Spondweni virus infection and pathogenesis in mice. Cell Rep 26:1585–1597. doi: 10.1016/j.celrep.2019.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gorman MJ, Caine EA, Zaitsev K, Begley MC, Weger-Lucarelli J, Uccellini MB, Tripathi S, Morrison J, Yount BL, Dinnon KH, Rückert C, Young MC, Zhu Z, Robertson SJ, McNally KL, Ye J, Cao B, Mysorekar IU, Ebel GD, Baric RS, Best SM, Artyomov MN, Garcia-Sastre A, Diamond MS. 2018. An immunocompetent mouse model of Zika virus infection. Cell Host Microbe 23:672–685. doi: 10.1016/j.chom.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Plante KS, Plante JA, Azar SR, Shinde DP, Scharton D, Versiani AF, Oliveira da Silva NI, Strange T, Sacchetto L, Fokam EB, Rossi SL, Weaver SC, Marques RE, Nogueira ML, Vasilakis N. 2024. Potential of Ilhéus virus to emerge. Heliyon 10:e27934. doi: 10.1016/j.heliyon.2024.e27934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. de Barros VED, Saggioro FP, Neder L, de Oliveira França RF, Mariguela V, Chávez JH, Penharvel S, Forjaz J, da Fonseca BAL, Figueiredo LTM. 2011. An experimental model of meningoencephalomyelitis by rocio flavivirus in BALB/C mice: inflammatory response, cytokine production, and histopathology. Am J Trop Med Hyg 85:363–373. doi: 10.4269/ajtmh.2011.10-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Amarilla AA, Santos-Junior NN, Figueiredo ML, Luiz JPM, Fumagalli MJ, Colón DF, Lippi V, Alfonso HL, Lima-Junior DS, Trabuco AC, et al. 2019. CCR2 plays a protective role in rocio virus–induced encephalitis by promoting macrophage infiltration into the brain. J Infect Dis:2015–2025. doi: 10.1093/infdis/jiz029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Macdonald F. 1952. Murray Valley encephalitis infection in the laboratory mouse. I. Influence of age on susceptibility to infection. Aust J Exp Biol Med Sci 30:319–324. doi: 10.1038/icb.1952.29 [DOI] [PubMed] [Google Scholar]
- 122. Licon Luna RM, Lee E, Müllbacher A, Blanden RV, Langman R, Lobigs M. 2002. Lack of both fas ligand and perforin protects from flavivirus-mediated encephalitis in mice. J Virol 76:3202–3211. doi: 10.1128/jvi.76.7.3202-3211.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mcminn PC, Dalgarno L, Weir RC. 1996. A comparison of the spread of Murray Valley encephalitis viruses of high or low neuroinvasiveness in the tissues of swiss mice after peripheral inoculation. Virology (Auckl) 220:414–423. doi: 10.1006/viro.1996.0329 [DOI] [PubMed] [Google Scholar]
- 124. Matthews V, Robertson T, Kendrick T, Abdo M, Papadimitriou J, McMinn P. 2000. Morphological features of Murray Valley encephalitis virus infection in the central nervous system of swiss mice. Int J Exp Pathol 81:31–40. doi: 10.1046/j.1365-2613.2000.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Andrews DM, Matthews VB, Sammels LM, Carrello AC, McMinn PC. 1999. The severity of Murray Valley encephalitis in mice is linked to neutrophil infiltration and inducible nitric oxide synthase activity in the central nervous system. J Virol 73:8781–8790. doi: 10.1128/JVI.73.10.8781-8790.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kroeger MA, McMinn PC. 2002. Murray Valley encephalitis virus recombinant subviral particles protect mice from lethal challenge with virulent wild-type virus. Arch Virol 147:1155–1172. doi: 10.1007/s00705-002-0809-3 [DOI] [PubMed] [Google Scholar]
- 127. Lobigs M, Müllbacher A, Wang Y, Pavy M, Lee E. 2003. Role of type I and type II interferon responses in recovery from infection with an encephalitic flavivirus. J Gen Virol 84:567–572. doi: 10.1099/vir.0.18654-0 [DOI] [PubMed] [Google Scholar]
- 128. Kuchinsky SC, Frere F, Heitzman-Breen N, Golden J, Vázquez A, Honaker CF, Siegel PB, Ciupe SM, LeRoith T, Duggal NK. 2021. Pathogenesis and shedding of Usutu virus in juvenile chickens. Emerg Microbes Infect 10:725–738. doi: 10.1080/22221751.2021.1908850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chvala S, Kolodziejek J, Nowotny N, Weissenböck H. 2004. Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J Comp Pathol 131:176–185. doi: 10.1016/j.jcpa.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 130. Chung YS. 1970. Viraemia and antibody response in chickens infected with arboviruses. J Comp Pathol 80:311–314. doi: 10.1016/0021-9975(70)90100-3 [DOI] [PubMed] [Google Scholar]
- 131. Patiris PJ, Oceguera LF, Peck GW, Chiles RE, Reisen WK, Hanson CV. 2008. Serologic diagnosis of West Nile and St. Louis encephalitis virus infections in domestic chickens. Am J Trop Med Hyg 78:434–441. doi: 10.4269/ajtmh.2008.78.434 [DOI] [PubMed] [Google Scholar]
- 132. Langevin SA, Bunning M, Davis B, Komar N. 2001. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg Infect Dis 7:726–729. doi: 10.3201/eid0704.017422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chvala S, Bakonyi T, Hackl R, Hess M, Nowotny N, Weissenböck H. 2005. Limited pathogenicity of Usutu virus for the domestic chicken ( Gallus domesticus) . Avian Pathol J WVPA 34:392–395. doi: 10.1080/03079450500268500 [DOI] [PubMed] [Google Scholar]
- 134. Chvala S, Bakonyi T, Hackl R, Hess M, Nowotny N, Weissenböck H. 2006. Limited pathogenicity of Usutu virus for the domestic goose ( Anser anser f. domestica ) following experimental inoculation. J Vet Med Ser B 53:171–175. doi: 10.1111/j.1439-0450.2006.00942.x [DOI] [PubMed] [Google Scholar]
- 135. Roiz D, Vázquez A, Ruiz S, Tenorio A, Soriguer R, Figuerola J. 2019. Evidence that passerine birds act as amplifying hosts for Usutu virus circulation. Ecohealth 16:734–742. doi: 10.1007/s10393-019-01441-3 [DOI] [PubMed] [Google Scholar]
- 136. Marzal A, Ferraguti M, Muriel J, Magallanes S, Ortiz JA, García-Longoria L, Bravo-Barriga D, Guerrero-Carvajal F, Aguilera-Sepúlveda P, Llorente F, de Lope F, Jiménez-Clavero MÁ, Frontera E. 2022. Circulation of zoonotic flaviviruses in wild passerine birds in Western Spain. Vet Microbiol 268:109399. doi: 10.1016/j.vetmic.2022.109399 [DOI] [PubMed] [Google Scholar]
- 137. Benzarti E, Rivas J, Sarlet M, Franssen M, Desmecht D, Schmidt-Chanasit J, Savini G, Lorusso A, Van Laere A-S, Garigliany M-M. 2020. Experimental Usutu virus infection in domestic canaries Serinus canaria. Viruses 12:164. doi: 10.3390/v12020164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hofmeister EK, Lund M, Shearn Bochsler V. 2018. West Nile virus infection in American singer canaries: an experimental model in a highly susceptible avian species. Vet Pathol 55:531–538. doi: 10.1177/0300985818760377 [DOI] [PubMed] [Google Scholar]
- 139. Monath TP, Kemp GE, Cropp CB, Bowen GS. 1978. Experimental infection of house sparrows (Passer domesticus) with rocio virus. Am J Trop Med Hyg 27:1251–1254. doi: 10.4269/ajtmh.1978.27.1251 [DOI] [PubMed] [Google Scholar]
- 140. Mitchell CJ, Monath TP, Cropp CB. 1981. Experimental transmission of rocio virus by mosquitoes. Am J Trop Med Hyg 30:465–472. doi: 10.4269/ajtmh.1981.30.465 [DOI] [PubMed] [Google Scholar]
- 141. Coetzer JA. 1977. The pathology of Rift valley fever. I. Lesions occurring in natural cases in new-born lambs. Onderstepoort J Vet Res 44:205–211. [PubMed] [Google Scholar]
- 142. Oymans J, van Keulen L, Wichgers Schreur PJ, Kortekaas J. 2020. Early pathogenesis of Wesselsbron disease in pregnant ewes. Pathogens 9:373. doi: 10.3390/pathogens9050373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ribeiro MR, Moreli JB, Marques RE, Papa MP, Meuren LM, Rahal P, de Arruda LB, Oliani AH, Oliani DCMV, Oliani SM, Narayanan A, Nogueira ML. 2018. Zika-virus-infected human full-term placental explants display pro-inflammatory responses and undergo apoptosis. Arch Virol 163:2687–2699. doi: 10.1007/s00705-018-3911-x [DOI] [PubMed] [Google Scholar]
- 144. Baba SS, Fagbami AH, Olaleye OD. 1988. Clinical and pathological responses of West African dwarf goats (Fouta Djallon) infected with Nigerian strain of Wesselsbron virus. Rev Elev Med Vet Pays Trop 41:329–335. [PubMed] [Google Scholar]
- 145. Baba SS. 1993. Virological and immunological studies of Wesselsbron virus in experimentally infected red Sokoto (Maradi) goats. Vet Microbiol 34:311–320. doi: 10.1016/0378-1135(93)90056-D [DOI] [PubMed] [Google Scholar]
- 146. Venter M, van Eeden C, Williams J, van Niekerk S, Steyl J, Jooste T, Swanepoel R. 2014. Arboviruses associated with neurological disease in animals in South Africa and their zoonotic potential in humans. Int J Infect Dis 21:184. doi: 10.1016/j.ijid.2014.03.804 [DOI] [Google Scholar]
- 147. Kay BH, Pollitt CC, Fanning ID, Hall RA. 1987. The experimental infection of horses with Murray Valley encephalitis and ross river viruses. Aust Veterinary J 64:52–55. doi: 10.1111/j.1751-0813.1987.tb16129.x [DOI] [PubMed] [Google Scholar]
- 148. Bażanów B, Jansen van Vuren P, Szymański P, Stygar D, Frącka A, Twardoń J, Kozdrowski R, Pawęska JT. 2018. A survey on West Nile and Usutu viruses in horses and birds in Poland. Viruses 10:87. doi: 10.3390/v10020087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Schvartz G, Tirosh-Levy S, Erester O, Shenhar R, Levy H, Bazanow B, Gelman B, Steinman A. 2020. Exposure of horses in Israel to West Nile virus and Usutu virus. Viruses 12:1099. doi: 10.3390/v12101099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Laidoudi Y, Durand G, Watier-Grillot S, Dessimoulie A-S, Labarde C, Normand T, Andréo V, Guérin P, Grard G, Davoust B. 2023. Evidence of antibodies against the West Nile virus and the Usutu virus in dogs and horses from the SouthEast of France. Transbound Emerg Dis 2023:1–8. doi: 10.1155/2023/8779723 [DOI] [Google Scholar]
- 151. Magallanes S, Llorente F, Ruiz-López MJ, Martínez-de la Puente J, Soriguer R, Calderon J, Jímenez-Clavero MÁ, Aguilera-Sepúlveda P, Figuerola J. 2023. Long-term serological surveillance for West Nile and Usutu virus in horses in South-West Spain. One Health 17:100578. doi: 10.1016/j.onehlt.2023.100578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McCormack WT, Tjoelker LW, Thompson CB. 1991. Avian B-cell development: generation of an immunoglobulin repertoire by gene conversion. Annu Rev Immunol 9:219–241. doi: 10.1146/annurev.iy.09.040191.001251 [DOI] [PubMed] [Google Scholar]
- 153. Wigley P. 2017. Immunology of birds, p 1–8. In In Encyclopedia of life sciences. John Wiley & Sons, Ltd. [Google Scholar]
- 154. Mackay CR, Beya MF, Matzinger P. 1989. γ/δ T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol 19:1477–1483. doi: 10.1002/eji.1830190820 [DOI] [PubMed] [Google Scholar]
- 155. Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. 2019. Global expansion and redistribution of aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis 13:e0007213. doi: 10.1371/journal.pntd.0007213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Massaro E, Kondor D, Ratti C. 2019. Assessing the interplay between human mobility and mosquito borne diseases in urban environments. Sci Rep 9:16911. doi: 10.1038/s41598-019-53127-z [DOI] [PMC free article] [PubMed] [Google Scholar]