Table 4.
Lipinski’s rule of 5 violations and ADME properties of PH011669 and its optimised version.
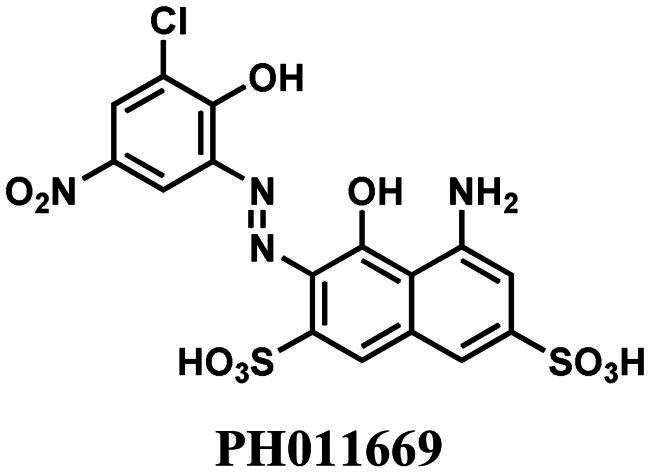
|
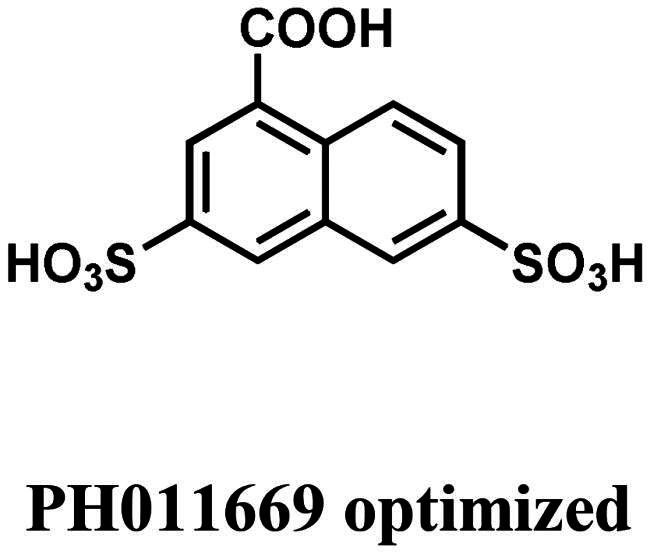
|
|
|---|---|---|
| Lipinski’s rule of 5 | ||
| 150 Da ≤ MW ≤500 Da | 518.9 Da | 332.3 Da |
| −0.7 ≤ Log P ≤ 5 | 2.06 | 0.19 |
| ≤10 H-bond acceptors | 12 | 8 |
| ≤5 H-bond donors | 5 | 3 |
| Other pharmacokinetic properties | ||
| 20 Å2 ≤ TPSA ≤150 Å2 | 262.5 Å2 | 162.8 Å2 |
| −6 ≤ Log S ≤ 0 | −4.38 | −2.17 |
| No. of rotatable bonds ≤ 7a | 5 | 3 |
| Presence of azo group | Yes | No |
| Gastrointestinal absorptionb | Low | Low |
| Interactions with CYP isoenzymesc | No | No |
MW: molecular weight; TPSA: topological polar surface area; CYP: cytochromes P450; BBB: blood-brain barrier.
aDrugs with more than seven rotatable bonds are flexible and can lead to unfavourable ADME properties, as the increased conformational entropy can distort target binding.
bFor a drug to be effective, its gastrointestinal absorption should be high to enter the bloodstream.
cAssessing the inhibitory interactions of a drug with the CYP isoenzymes CYP1A2, CYP2C19, CYP2C9, CYP2D6 and CYP3A4 is essential because inhibition of these isoenzymes can lead to drug–drug interactions, increasing the risk of adverse effects. In this category, “no” means that the drug is not an inhibitor of any CYP isoenzyme.
