FIGURE 3.
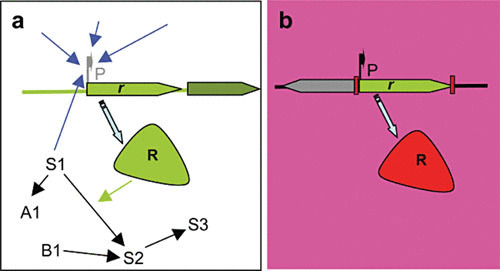
Exaptation and gene decontextualization in the evolution of antibiotic resistance. Antibiotic resistance genes (r) have evolved for millions of years located in the chromosomes of their original hosts (a). During this evolution, the expression of these determinants (R) from their promoters (P) has been finely tuned to respond to several signals that might include the response to environmental and metabolic changes (blue arrows). Besides, the determinants encoded by these genes are integrated in physiological networks, where they can play a role as metabolic enzymes. S1 to S3 represent metabolites of the same pathway, and A1 and B1 metabolites of other interconnected pathways. When these genes are integrated in gene capture (for instance, an integron) and transfer units (for instance, a plasmid), they can be transferred to a new host and submitted to strong antibiotic selective pressure (b), and they can be constitutively expressed from a strong promoter (P) present in the capture unit and therefore lack the regulatory and physiological network encountered in the original host (gene decontextualization). Under these circumstances, the only function these determinants can play is antibiotic resistance, in such a way that this functional shift is not the consequence of adaptive changes in the determinants but rather of changes in their environment (exaptation). Reproduced with permission from reference 127.
