FIGURE 1.
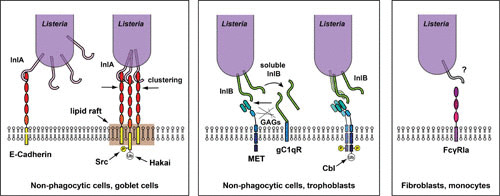
Cellular receptors for L. monocytogenes in host cells. The receptor for InlA in nonphagocytic polarized cells (including goblet cells) is the transmembrane molecule E-cadherin. Interaction takes place between the InlA leucine-rich repeats (LRRs) and the first extracellular domain of E-cadherin, leading to phosphorylation and ubiquitylation of the cytoplasmic domain of E-cadherin by the kinase Src and the ubiquitin ligase Hakai, respectively. Clustering of E-cadherin requires the presence of lipid rafts (left panel). Via its C-terminal glycine-tryptophan (GW) repeats, InlB interacts with the receptor for the globular part of the C1q complement component (gC1qR) and glycosaminoglycans, which enable interaction of the N-terminal LRRs of InlB with the tyrosine receptor kinase Met in nonphagocytic cells (including trophoblasts). Met dimerization upon interaction with InlB leads to autophosphorylation and recruitment of the ubiquitin ligase Cbl, which ubiquitylates the cytoplasmic tail of Met (center panel). In fibroblasts and monocytes, a function for the FcγRIA receptor has been described for L. monocytogenes internalization, via interaction with a still unidentified L. monocytogenes surface molecule (right panel). Modified from reference 12.
