FIGURE 2.
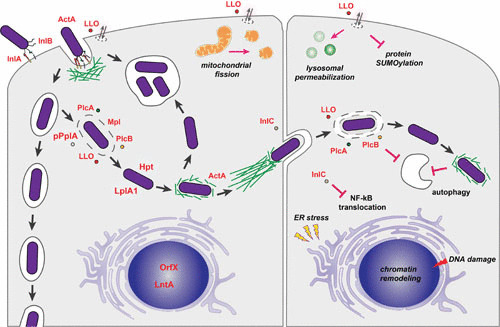
L. monocytogenes intracellular stages. L. monocytogenes is able to induce its entry into nonphagocytic cells mainly via the interaction of InlA and InlB with host cells receptors that promote actin recruitment, remodeling of the plasma membrane, and bacterial engulfment. The surface molecule ActA and the secreted pore-forming toxin LLO have also been implicated in the early L. monocytogenes entry steps (left cell, upper left). In goblet cells, upon internalization, L. monocytogenes is localized in a vacuole, and through transcytosis the bacterium is translocated to the lamina propria (left cell, left). In other cells, the combined activity of diverse virulence factors, including the pore-forming LLO, the metalloprotease Mpl, the phospholipases PlcA and PlcB, and the pheromone pPplA, favor disruption of the vacuole and L. monocytogenes release in the cytosol, where the bacteria takes advantage of host metabolites via the phosphate transporter Hpt and the lipoate protein ligase LplA. The surface protein ActA promotes actin-based motility, and the secreted protein InlC favors reduction of plasma membrane cortical tension, allowing L. monocytogenes to form protrusions and to invade neighboring cells. LLO and the phospholipases PlcA and PlcB contribute to the disruption of the double-membrane vacuole (right cell). L. monocytogenes has been observed in large spacious compartments that may arise rapidly after internalization of bacteria or upon decrease of ActA expression in already cytoplasmic bacteria (left cell, upper center). Extracellular LLO is able to modulate different cellular functions, including mitochondrial fission, lysosomal permeabilization, protein SUMOylation, ER stress, DNA damage, and chromatin remodeling. The phospholipases PlcA and PlcB, together with actin polymerization by ActA, have been implicated in the resistance to autophagy (195). The secreted molecule InlC prevents NF-κB translocation to the nucleus. Modified from reference 12.
