FIGURE 3.
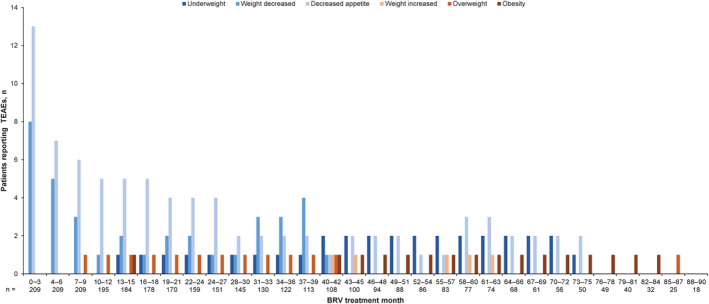
Incidence of weight change and appetite–related TEAEs during the BRV treatment period (SS).a BRV, brivaracetam; SS, safety set; TEAE, treatment‐emergent adverse event. aFigure presents the number of patients reporting TEAEs with a start date within the specified 3‐month time interval. Patients were included in a 3‐month interval if they were receiving BRV at any time during that interval. One month was defined as 30 days. No weight change or appetite–related TEAEs were reported after month 87.
