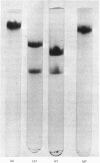
Abstract
The cleavage of human complement component C5 to fragment C5b by the alternative pathway C5 convertase was studied. The alternative-pathway C5 convertase on zymosan can be represented by the empirical formula zymosan--C3b2BbP. Both properdin-stabilized C3 and C5 convertase activities decay with a half life of 34 min correlating with the loss of the Bb subunit. The C5 convertase functions in a stepwise fashion: first, C5 binds to C3b and this is followed by cleavage of C5 to C5b. The capacity to bind C3b is a stable feature of component C5, as C5b also has this binding capacity. Component C5, unlike component C3, does not form covalent bonds with zymosan after activation, and C5 is not inhibited by amines. Therefore C5, although similar in structure to C3, does not appear to contain the internal thioester group reported for C3 and C4.
Full text
PDF

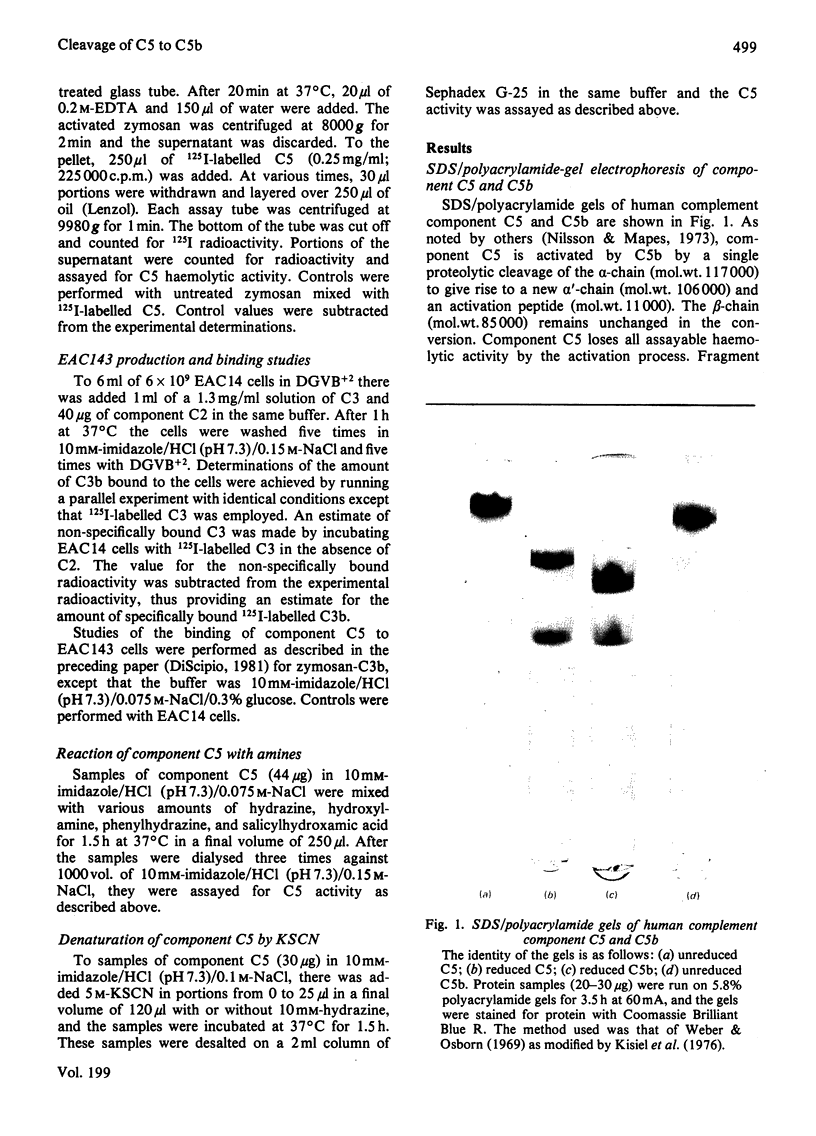
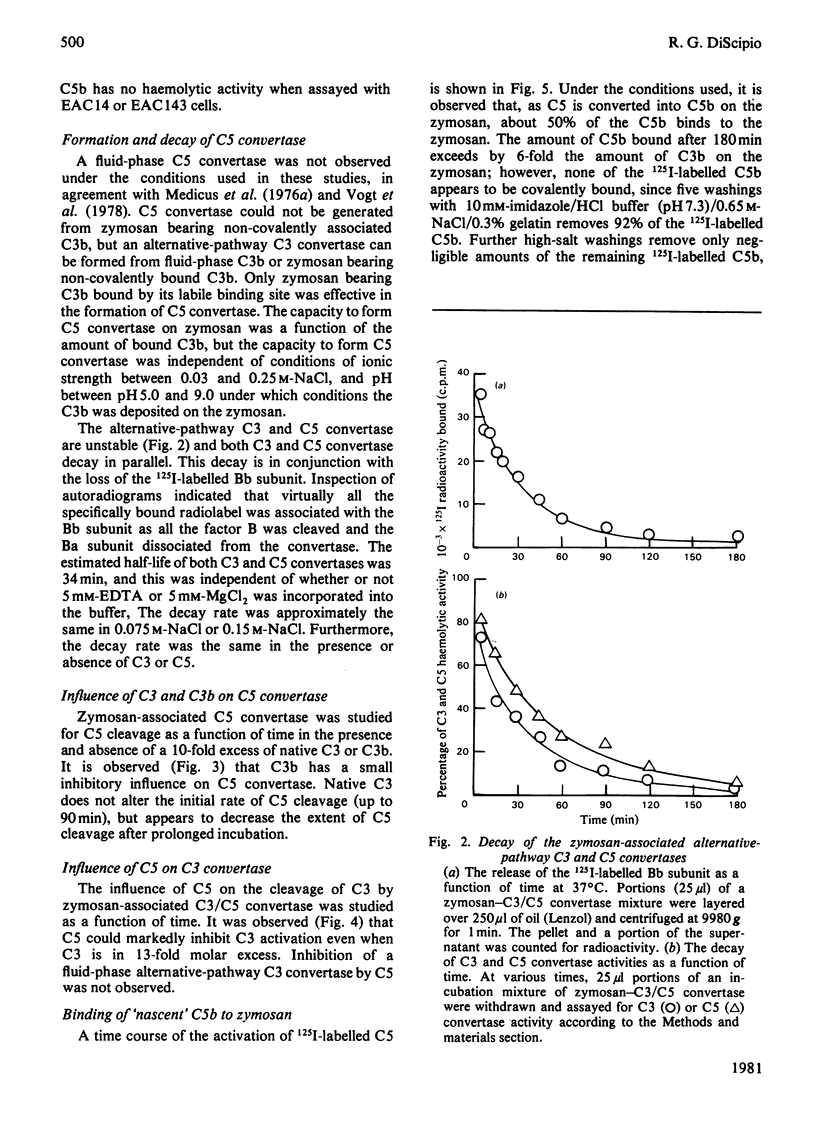
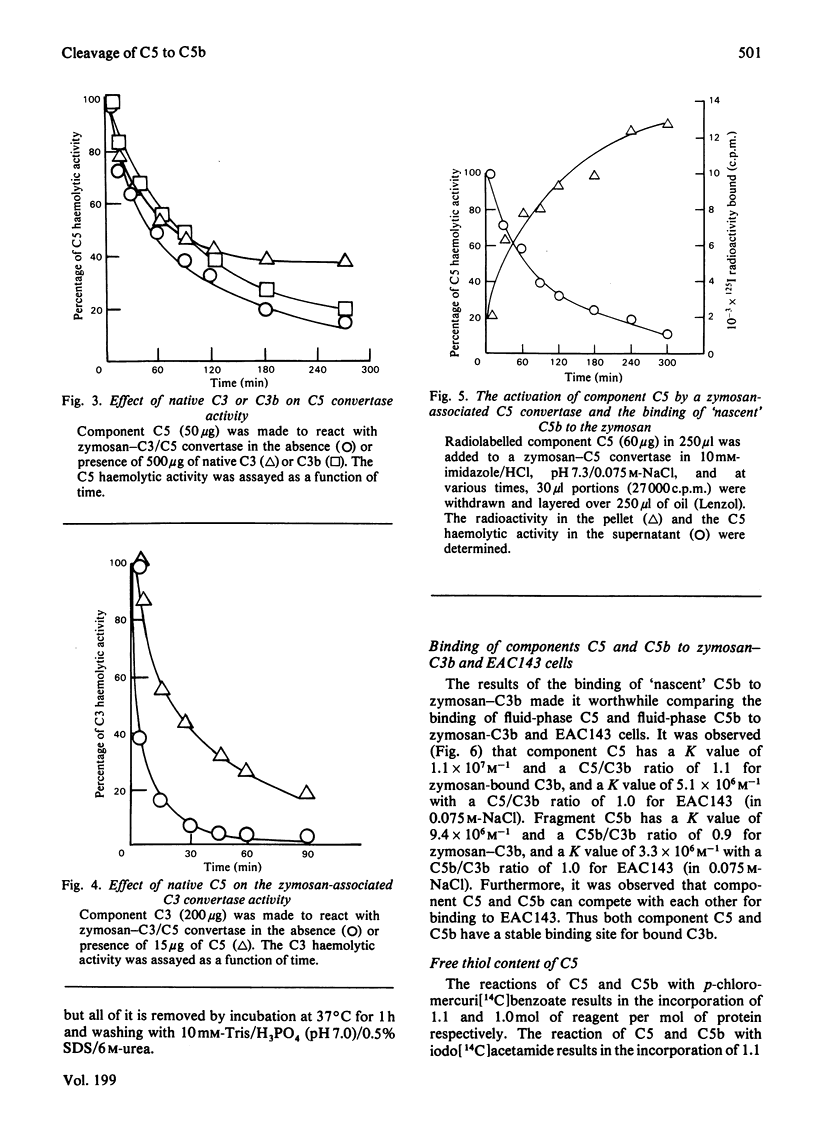


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade V., Lee G. D., Nicholson A., Shin H. S., Mayer M. M. The reaction of zymosan with the properdin system in normal and C4-deficienct guinea pig serum. Demonstration of C3- and C5-cleaving multi-unit enzymes, both containing factor B, and acceleration of their formation by the classical complement pathway. J Immunol. 1973 Nov;111(5):1389–1400. [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D. L., Gagnon J., Porter R. R. Partial sequence of human complement component factor B: novel type of serine protease. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4923–4927. doi: 10.1073/pnas.77.8.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiScipio R. G. The binding of human complement proteins C5, factor B, beta 1H and properdin to complement fragment C3b on zymosan. Biochem J. 1981 Dec 1;199(3):485–496. doi: 10.1042/bj1990485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978 Oct 10;253(19):6955–6964. [PubMed] [Google Scholar]
- Goldlust M. B., Shin H. S., Hammer C. H., Mayer M. M. Studies of complement complex C5b,6 eluted from--EAC-6: reaction of C5b,6 with EAC4b,3b and evidence on the role of C2a and C3b in the activation of C5. J Immunol. 1974 Sep;113(3):998–1007. [PubMed] [Google Scholar]
- Hammer C. H., Abramovitz A. S., Mayer M. M. A new activity of complement component C3: cell-bound C3b potentiates lysis of erythrocytes by C5b,6 and terminal components. J Immunol. 1976 Sep;117(3):830–834. [PubMed] [Google Scholar]
- Harpel P. C., Hayes M. B., Hugli T. E. Heat-induced fragmentation of human alpha 2-macroglobulin. J Biol Chem. 1979 Sep 10;254(17):8669–8678. [PubMed] [Google Scholar]
- Isenman D. E., Podack E. R., Cooper N. R. The interaction of C5 with C3b in free solution: a sufficient condition for cleavage by a fluid phase C3/C5 convertase. J Immunol. 1980 Jan;124(1):326–331. [PubMed] [Google Scholar]
- Janatova J., Lorenz P. E., Schechter A. N., Prahl J. W., Tack B. F. Third component of human complement: appearance of a sulfhydryl group following chemical or enzymatic inactivation. Biochemistry. 1980 Sep 16;19(19):4471–4478. [PubMed] [Google Scholar]
- Janatova J., Tack B. F., Prahl J. W. Third component of human complement: structural requirements for its function. Biochemistry. 1980 Sep 16;19(19):4479–4485. doi: 10.1021/bi00560a015. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Ericsson L. H., Davie E. W. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976 Nov 2;15(22):4893–4900. doi: 10.1021/bi00667a022. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Holcombe F. H., Levine R. P. Interaction between the labile binding sites of the fourth (C4) and fifth (C5) human complement proteins and erythrocyte cell membranes. J Immunol. 1980 Aug;125(2):634–639. [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- MUELLER-EBERHARD H. J., BIRO C. E. ISOLATION AND DESCRIPTION OF THE FOURTH COMPONENT OF HUMAN COMPLEMENT. J Exp Med. 1963 Sep 1;118:447–466. doi: 10.1084/jem.118.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicus R. G., Götze O., Müller-Eberhard H. J. Alternative pathway of complement: recruitment of precursor properdin by the labile C3/C5 convertase and the potentiation of the pathway. J Exp Med. 1976 Oct 1;144(4):1076–1093. doi: 10.1084/jem.144.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicus R. G., Götze O., Müller-Eberhard H. J. The serine protease nature of the C3 and C5 convertases of the classical and alternative complement pathways. Scand J Immunol. 1976;5(9):1049–1055. doi: 10.1111/j.1365-3083.1976.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Mole J. E., Niemann M. A. Structural evidence that complement factor B constitutes a novel class of serine protease. J Biol Chem. 1980 Sep 25;255(18):8472–8476. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H., PILLEMER L. The multiplicity of plasmin inhibitors in human serum, demonstrated by the effect of primary amino compounds. Bull Johns Hopkins Hosp. 1954 Apr;94(4):169–179. [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Barrett A. J. Covalent binding of proteinases in their reaction with alpha 2-macroglobulin. Biochem J. 1980 Jun 1;187(3):695–701. doi: 10.1042/bj1870695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Sim E. Autolytic fragmentation of complement components C3 and C4 under denaturing conditions, a property shared with alpha 2-macroglobulin. Biochem J. 1981 Jan 1;193(1):129–141. doi: 10.1042/bj1930129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Twose T. M., Paterson D. S., Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981 Jan 1;193(1):115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Fifth component of human complement: purification from plasma and polypeptide chain structure. Biochemistry. 1979 Apr 17;18(8):1490–1497. doi: 10.1021/bi00575a016. [DOI] [PubMed] [Google Scholar]
- Vogt W., Schmidt G., Von Buttlar B., Dieminger L. A new function of the activated third component of complement: binding to C5, an essential step for C5 activation. Immunology. 1978 Jan;34(1):29–40. [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]