Abstract
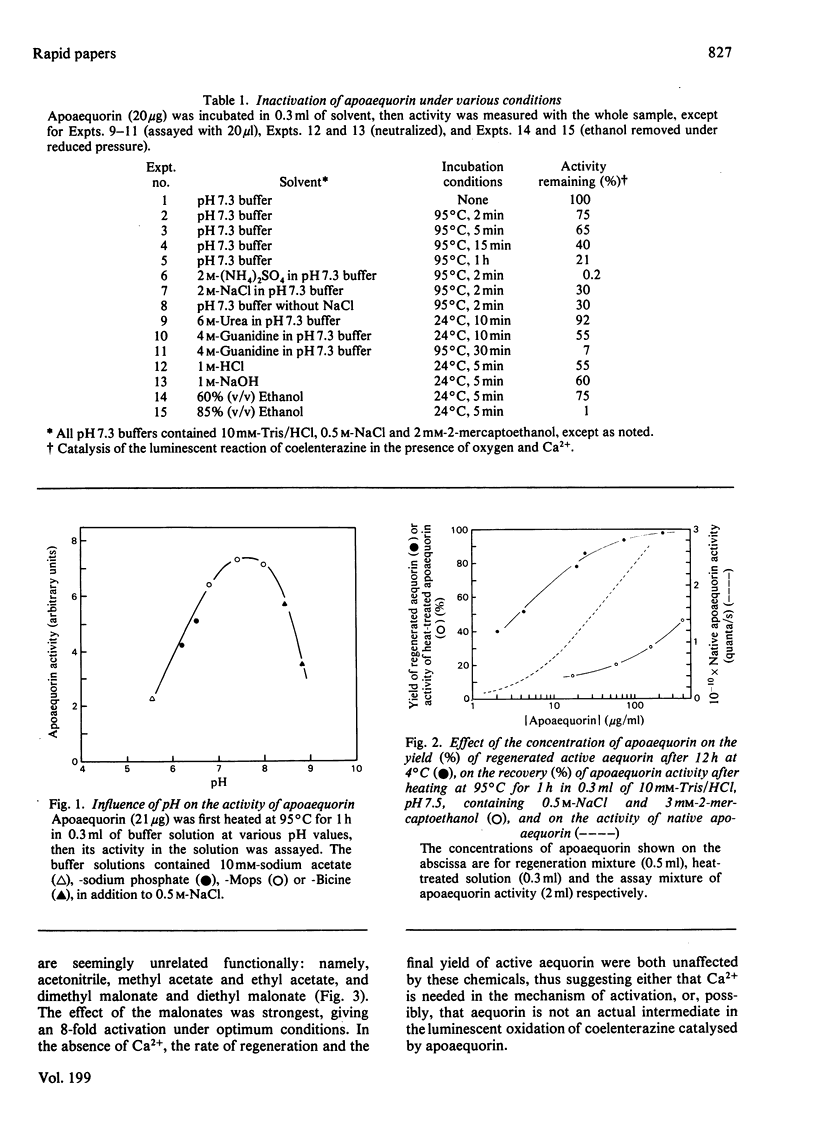
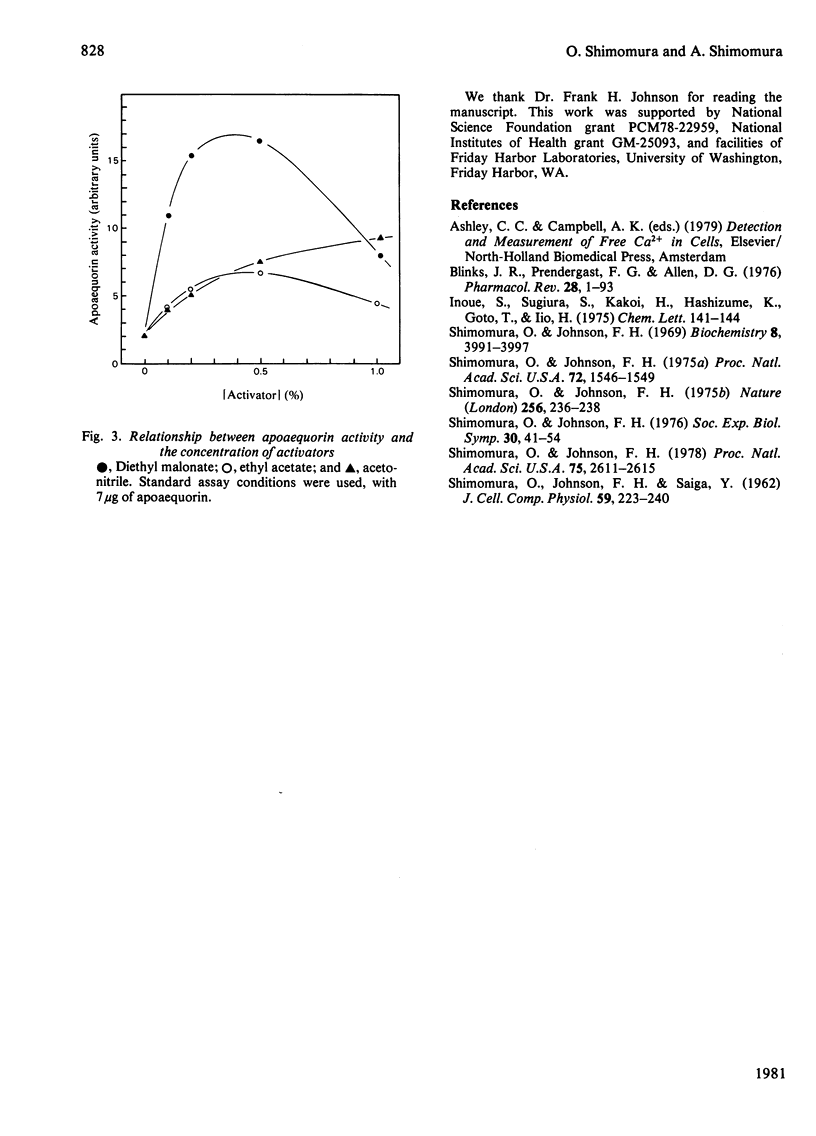
Although native aequorin is highly susceptible to inactivation, apoaequorin is highly resistant to various processes of denaturation. Apoaequorin was inactivated only partially at a temperature of 95 degrees C or by treatments with 6 M-urea, 4 M-guanidine hydrochloride, 1 M-HCl and 1 M-NaOH. It was nearly completely inactivated in 85% ethanol or by heating at 95 degrees C in 2 M-(NH4)2SO4, but over 50% of apoaequorin activity was restored in both cases merely by dissolving the coagulated protein in 4 M-guanidine hydrochloride. In the reconstitution of aequorin, partially inactivated apoaequorin yielded more aequorin than expected from the activity of the partially inactivated apoaequorin used, suggesting that the process of reconstitution promotes the renaturation of denatured apoaequorin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinks J. R., Prendergast F. G., Allen D. G. Photoproteins as biological calcium indicators. Pharmacol Rev. 1976 Mar;28(1):1–93. [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Chemical nature of bioluminescence systems in coelenterates. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1546–1549. doi: 10.1073/pnas.72.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Peroxidized coelenterazine, the active group in the photoprotein aequorin. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2611–2615. doi: 10.1073/pnas.75.6.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Properties of the bioluminescent protein aequorin. Biochemistry. 1969 Oct;8(10):3991–3997. doi: 10.1021/bi00838a015. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Regeneration of the photoprotein aequorin. Nature. 1975 Jul 17;256(5514):236–238. doi: 10.1038/256236a0. [DOI] [PubMed] [Google Scholar]


